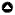The following is the established format for referencing this article:
Blight, L. K., W. O'Shea, and G. T. W. McClelland. 2022. A comparison of historical and contemporary reproductive traits in a declining population of Glaucous-winged Gulls (Larus glaucescens). Avian Conservation and Ecology 17(2):41.ABSTRACT
Understanding how organisms manage life history trade-offs under variable environmental conditions is an aim that is central to ecology. Comparing modern reproductive data with those from historical studies can increase understanding of the range of historical conditions that have acted on a given species over time. We use recent (2008-2010) and historical (1960s-1980s) reproductive data from a single study colony, Mandarte Island, Canada, to help understand the recent population declines experienced by the Glaucous-winged Gull (Larus glaucescens) in North America's Salish Sea, a highly urbanized area. Because this long-lived species has also undergone regional long-term declines in reproductive investment via decreasing egg and clutch size, we assessed whether modern reproductive outcomes were similarly affected. Although our statistical power was hampered by small sample sizes, it appears that hatching success declined over time, from 0.83 (1960s) and 0.76 (1970s-1980s) to 0.60 (2008-2010). An apparent decline in nesting success, from 0.63 (1962) to 0.52 (this study), was not statistically significant. Recent clutch sizes showed intra-seasonal declines, a pattern unchanged from historical trends. In contrast, egg mass historically was constant within a given nesting season, but recent egg-mass data show intra-seasonal declines. We conclude that most gulls currently breeding on Mandarte Island cannot attain historical levels of reproductive success — perhaps because of declining environmental quality in the form of reduced availability of high-quality fish prey — although some high-quality pairs in this population are still able to maximize reproductive output. Our study highlights the importance of long-term study systems for identifying consequences of large-scale ecosystem changes; however, methodological clarity is essential to ensure data comparability through time. Ultimately, further study is needed to identify the drivers of reproductive changes in this population, something that could be used to inform future management decisions.RÉSUMÉ
INTRODUCTION
Understanding how organisms manage life history trade-offs to maximize reproductive output under variable environmental conditions is a central aim of ecology (Stearns 1976). Resource availability is arguably one of the most important determinants of reproductive success (Stearns 1992). In seabirds, energetic constraints on females due to low food availability or poor-quality food sources can limit clutch size (Sydeman et al. 1991), reduce offspring quality (Annett and Pierotti 1999), or delay the onset of breeding (Mills et al. 2008). Variation in resource availability may occur naturally, for example due to natural phenological cycles driving within-season variation in food availability (Cushing 1990); however, anthropogenic changes to the marine environment can also degrade resources (e.g., McKechnie et al. 2014) and consequently affect seabird reproductive success negatively over both the short and longer term (Annett and Pierotti 1999, Cury et al. 2011).
In North America’s Salish Sea, a coastal body of water adjacent to several large urban areas, there have been historical declines in the availability of high quality fish prey such as Pacific herring (Clupea pallasi) and other forage fish species (Wallace 1998, Therriault et al. 2009); since the 1980s, there have been reductions in duration and distribution of local herring spawning events, as well as in fish size-at-age (Therriault et al. 2009, Boldt et al. 2021). For Glaucous-winged Gulls (Larus glaucescens), this may have contributed to a documented long-term decline of fish in gull diet, which in turn has likely led to the decreases in Glaucous-winged Gull reproductive investment and population size that have occurred over recent decades (Blight 2011, Blight et al. 2015a,b, Blight et al. 2019). Although Larus gulls are well-known as generalist foragers, greater reproductive success and improved body condition in the genus have been linked with access to high-quality food (e.g., Ward 1973, Annett and Pierotti 1999, Hebert et al. 2002), as has egg production specifically (Hiom et al. 1991, Bolton et al. 1993). Long-term regional declines in average clutch size and egg size, as well as delayed onset of breeding, have occurred in Glaucous-winged Gulls over the last century, coinciding with a gradual shift since the 1800s to a diet that has included less fish prey (Blight 2011, Blight et al. 2015b, Hobson et al. 2015). Since the 1980s, gull diet also appears to have shifted from Pacific herring to Pacific sand lance (Ammodytes hexapterus), at least for the two colonies on the West Coast of Vancouver Island and in the Salish Sea where modern diet has been examined in detail (Davis et al. 2015). The documented decline in egg production has likely contributed to the steep population declines observed in recent decades, with the number of Glaucous-winged Gulls nesting in the Salish Sea in 2010 at about 50% of numbers recorded in 1986 (with those peak counts likely resulting from cessation of human persecution earlier in the 1900s; Reid 1988, Vermeer and Devito 1989, Blight et al. 2015a).
Because these changes in reproduction are potentially driving regional population declines, it is of interest to explore them further, via historical comparisons of other aspects of the Glaucous-winged Gull reproductive cycle. Although egg production in this population has shown long-term declines, it is not clear whether this decreased egg output has merely resulted in the production of commensurately fewer chicks, or if other aspects of the reproductive cycle have shown similar decreases, compounding negative effects. Any such downstream effects could be direct, e.g., with smaller eggs resulting in reduced hatch success (Martin 1987), or additive, e.g., with the environmental drivers of smaller egg and clutch sizes also contributing to decreased survival of offspring to fledging age (Annett and Pierotti 1999). Conversely, such drivers could result in adults expending more energy to rear the same number of offspring and potentially experiencing lower survival rates post-breeding (Reid 1987a, Annett and Pierotti 1999). Similarly, declines in egg production could be a general indication of condition within the population, with birds unable to reach historical body condition pre-breeding, something that has the potential to influence all aspects of population life history (Chastel et al. 1995).
Earlier studies of Glaucous-winged Gulls in this region, and of gull populations elsewhere, have also shown decreases in clutch size within a season, with three-egg clutches typical of many Larus gulls occurring more frequently early in the season (Verbeek 1986, Sydeman et al. 1991). This is to be anticipated because evidence suggests that poorer-quality females who take longer to reach optimal laying condition lay smaller clutches and do so later in the season (e.g., Houston et al. 1983). Gull fledging success may also exhibit a seasonal decline (Sydeman et al. 1991). In contrast, Glaucous-winged Gull egg mass does not typically decline with lay date (Verbeek 1986).
Here we investigate whether documented declines in Glaucous-winged Gull reproductive investment (defined here as clutch size and egg size) over the past century are also evident among downstream reproductive outcomes in the Salish Sea. Because long-term egg and clutch size declines are thought to have been driven by a documented shift in diet (reduced access to higher quality foods; Blight et al. 2015b, Hobson et al. 2015) and as some intra-seasonal patterns in reproduction (i.e., trends in egg mass and the related metric of chick mass at hatch; clutch size or fledging success vs. lay date) may also be influenced by factors influencing diet, we investigated whether the historical intra-seasonal patterns in these parameters have been conserved in this population. Furthermore, because egg size can affect egg survival (Martin 1987) and post-hatch chick survival, a relationship particularly well-documented in gulls (Parsons 1970, Bolton 1991), we hypothesized that modern measurements of reproductive outcomes such as hatching success and fledging rate would also be significantly lower than the historical measures recorded for this region.
Overall, we wished to take advantage of a series of data from a single large colony, collected intermittently over approximately five decades (between 1963 and 2010), to investigate whether and how additional aspects of reproductive output may have changed over time in this population of Glaucous-winged Gulls. In the face of anthropogenic changes that alter the breeding environment, bird populations often exhibit changes to their reproductive strategies, which can reflect environmental stressors (e.g., Real et al. 2017). Ultimately, directing management activities to address declining trends in wild bird species requires a detailed understanding of these declines, and on which aspects of a species’ life history any environmental stressors may be acting.
METHODS
Study site and modern field data collection
Reproductive data were collected at the large (> 1000 pairs; Vermeer and Devito 1989, Blight 2014) Glaucous-winged Gull colony on Mandarte Island, Canada (48° 37’ 59” N, 123° 16’ 59” W). Data collection occurred from the pre-lay period through to chick fledge during the 2008, 2009, and 2010 breeding seasons (data collection dates: 30 April-18 August 2008; 13 May-30 August 2009; 13 May-20 August 2010).
Once the laying period began each season (est. 21-23 May; Blight 2011), we searched the colony daily for new nests, i.e., those in which laying had just commenced. Because we wanted to record reproductive parameters equally throughout the season, we continued searching for new nests each day until the daily search effort required to find 5 new nests abruptly increased from about 1.5-2 h to 4 h in late June (28 June, 2008; 25 June, 2009; 21 June, 2010). Each new nest was marked with a numbered flag and checked every 1-2 d (based on the species’ laying interval of approximately 2 d; Hayward and Verbeek 2020; LKB unpublished data) until clutch completion. Upon discovery, each new egg was weighed (g; Pesola balance) to estimate egg mass, and both length and width (mm; dial caliper) were measured. Eggs were also labeled with a permanent marker to denote lay order (A, B, C; D if required; see Results). In cases in which nests were visited after two eggs had been laid and order was not possible to discern, both new eggs were labeled AB if there were no eggs of known lay order in the nest and BC if an egg had been previously labeled in the nest. Though Glaucous-winged Gulls typically produce three-egg clutches, not all pairs do so. Thus, for the nest-check period, if no new egg appeared for seven days, we considered that clutch to be complete and stopped that nest check until the hatch period. This protocol meant that any missing eggs were detectable, i.e., true clutch size was recorded.
Mean incubation period from clutch commencement is about 28 d in this species (Verbeek 1986). To determine hatching success (number of chicks hatched per number of eggs laid), we visited each nest every 2-3 d at hatch commencement, beginning 1 day prior to the estimated hatch date of the clutch’s first-laid egg. Each hatching event was followed until the chick had fully emerged from the egg. For each newly hatched chick (< 1 d of age), we measured mass (g; Pesola balance), wing chord (mm; wing rule), and tarsus length (mm; dial caliper). After we recorded data, we marked individuals with a dab of nail polish on the head to avoid taking repeat measurements. Chicks found on rainy days were not measured in order to reduce impacts of handling.
We carried out nest searches and nest checks using approaches designed to limit disturbance at the colony. Specifically, on daily rounds, 1 to 2 observers slowly entered each gull “meadow” (open areas of rocky and grassy terrain screened by tall shrubs, the primary nesting habitat on Mandarte Island), then waited quietly at the meadow’s perimeter for nesting birds to settle prior to our commencing work. We minimized the amount of time spent in each meadow by selecting the shortest possible routes among study nests and visiting meadows a maximum of once per day. Rather than concentrating effort on smaller areas within the colony, study nests were drawn from meadows across the island to reduce observer time spent in each area. Whenever working in the meadows, we moved slowly and spoke quietly. Overall, this approach ensured that if disturbed, incubating or brooding birds only left their nests for a few minutes at a time and stayed nearby. Nests in open rocky areas or near cliffs were not selected for study nests because young chicks hatched in habitats without vegetation for hiding can flee long distances when disturbed, and may run off cliffs or be killed by adult birds in adjacent territories (Vermeer 1963, Hunt and Hunt 1976, Vermeer et al. 1988). This schedule and approach were conservative compared to earlier studies, in which nests were checked as frequently as three times per day in all habitats (Vermeer 1963) or daily from laying to hatch (Verbeek 1986).
An estimate of fledging success (defined as mean number of chicks fledged per chicks hatched per nest) was obtained by following a subset of nests through to fledging. Nests in this subsample were selected at chick hatch from study nests initiated across the breeding season, to a maximum of 50 nests. The criteria for selection were that (1) the nest had hatched at least one chick, and (2) the nest was clearly observable from a distant vantage point not visible to birds at the nest, to avoid chicks hiding from an observer and going uncounted. Chicks were considered fledged when they reached 30 d of age, following Vermeer’s (1963) demonstration that virtually all (92%) chick mortality occurs within the first 4 weeks of life (n = 2 years). Starting at est. d 30 post hatch, we observed fledging success nests with binoculars from a distance over one or more days until observers were satisfied the full complement of surviving chicks was accounted for. Because fledging success was calculated only from nests in which one or more chicks had hatched (i.e., study nests that had failed pre hatch were not part of this sample), we used the proportion of failed pre-hatch nests to derive sample size for annual estimates of nesting success (proportion of eggs laid that hatch and survive to become fledglings; Ricklefs 1973).
 |
(1) |
where NS is nesting success in year i, n is the number of chicks fledged in the fledging success cohort, p is the proportion of failed nests in the study sample, f is the number of nests in the fledging success cohort, and cs is mean clutch size for the study.
Historical data on reproductive parameters
We reviewed published studies of Glaucous-winged Gulls on Mandarte Island, searching for data on reproductive parameters of interest including hatching success, fledging success, and nesting success from the 1960s through the 1980s. We excluded historical studies of urban gulls nesting in nearby cities in the region (Hooper 1988, Vermeer et al. 1988) from our analyses because open rooftop habitats leave nests particularly vulnerable to observer disturbance, presumably affecting reproductive output (and reported results) in various undocumented ways. Other non-urban gull colonies in the area have not been the subject of reproductive studies. Thus, criteria for study selection were that reproductive data were collected on Mandarte Island, were clearly reported, and were not pooled across years. In some cases, only a subset of historical data was available. Specifically, Verbeek et al. (1986) reported hatching success for clutches of two and three eggs. Because one-egg clutches represented less than 10% of nests on the colony that year, and reported hatching success appeared to be similar to previously recorded data on Mandarte (e.g., Vermeer 1963), we opted to include these records to maximize our dataset. When investigating historical records of nesting success from Mandarte, we discovered a discrepancy between the values derived for nesting success from 1962, i.e., Vermeer (1963) = 0.58 and Hayward and Verbeek (2020) = 0.63. We opted to use the most recent statistic for our analyses, assuming it had been corrected from the earlier value; however, using the value from Vermeer (1963) produced similar results. We excluded Vermeer’s (1963) 1961 data for nesting success and hatching success because the author suggested that observer behavior (disturbance) likely affected reproductive success in that year.
Statistical analysis
We used linear models to assess intra-annual differences in Glaucous-winged Gull reproductive output for the years 2008 through 2010. Given our hypothesized patterns (see Introduction), we analyzed within-year trends for clutch size, chick mass (g) at hatch, and fledging success against day of year. We first analyzed intra-annual trends for each year, and if there was no within-year difference, we combined data from 2008-2010. For clutch size, a visual inspection of the data suggested a curvilinear trend in mean daily values — in part due to the values being bounded by the upper clutch size limit of three eggs — so for this parameter we used a quadratic term (a cubic model did not provide a better fit). Because egg size has a well-known tendency to be strongly correlated with chick mass in precocial species such as gulls (e.g., Parsons 1970, Grant 1991, Kilpi et al. 1996), we also assessed within-season egg mass trends to validate chick mass results. To control for factors that may also influence egg size and determine if egg size was related to lay date, we ran a linear mixed-effects model with egg mass (g) as the dependent variable and egg lay date, year (2008 set to 0), egg order (A was set to 0), and clutch size as fixed factors. Nest ID was included as a random effect to control for within nest effects.
To determine if rates of hatching success differed between historical and contemporary records on Mandarte Island, we fitted a binomial generalized linear model, with annual hatching success (annual proportion of eggs hatched per eggs laid) as the response variable and year as a fixed factor. To analyze declines in nesting success, we fitted a linear model with the annual proportion of eggs that survived to fledge as the response variable, and year as a fixed effect. We acknowledge that the power of these statistical tests is limited because of small sample sizes. All analyses were performed in R (R Core Team 2020), and figures were produced using the ggplot2 package (Wickham 2016).
RESULTS
Modern reproductive data
We monitored a total of 567 Glaucous-winged Gull nests over the three years of our field study (n = 232, 2008; n = 184, 2009; n = 151, 2010), discarding nests from our initial sample if nest markers were lost or nest fate was otherwise uncertain. This resulted in a total of 532 nests measured for hatching success (n = 207, 2008; n = 175, 2009; n = 150, 2010), and 354 chicks measured for chick mass (n = 119, 2008; n = 141, 2009; n = 94, 2010). For fledging success estimates, we followed 47 nests in 2008, 40 in 2009, and 50 in 2010. Interestingly, over the three years of our study we recorded among our study nests one four-egg clutch (2010; nest failed pre-hatch) and one supra-normal brood of four chicks successfully fledged by two parents at a single nest (2009).
As expected based on historical patterns, we observed a within-year decline in clutch size over each of the three years of our study (Fig. 1A; F2,671 = 115.39, p < 0.0001, all years combined), i.e., gulls that laid later in each season produced smaller clutches. The relationship of egg mass (g) to lay date was a negative one (LMM: β = -0.498, SE = 0.041, F1,868 = 145.81, p <0.001; Fig. 1B), and when we controlled for egg order, clutch size, and year, the negative relationship between egg mass and lay date still trended toward significance (LMM: β = -0.09, SE = 0.046, F1,606 = 33.49, p = 0.062). However, we did not find a similar within-year reduction in chick mass at hatch over the study by year, or for all years combined (F1,349 = 1.35, p = 0.25), with chick mass relatively constant across the breeding season (Fig. 1C). We found only weak evidence for intra-annual declines in fledge success over time, with one year (2009) showing a weak negative trend (F1,38 = 4.88, p = 0.03), and no temporal trends in 2008 (F1,45 = 0.24, p = 0.62) and 2010 (F1,48 = 1.19, p = 0.28; Fig. 1D). However, the 2009 trend was driven by late-in-season values not present in the 2008 and 2010 data (see Discussion).
Historical comparisons
Though several researchers have studied the breeding biology of this species in the Salish Sea (e.g., Vermeer 1963, Ward 1973, Hunt and Hunt 1976, Reid 1987a,b, 1988, Verbeek and Richardson 1982, Verbeek 1986, Vermeer et al. 1988, Vermeer and Devito 1989, Vermeer 1992), primarily at Mandarte Island, we found few useable datasets for our parameters of interest. Data from these earlier studies were generally collected or summarized in a way that rendered them incomparable to our modern data. Ultimately, only historical data for hatching success (1961, 1962, 1979, 1980) and nesting success (1962) were available for our comparisons. Hatch success showed a statistically significant decline from 0.83 (1962; Vermeer 1963) through a mean of 0.76 (1979 and 1980; Verbeek 1986) to one of 0.60 (2008-2010; this study; GLM: β = -0.023, SE = 0.002, F1,5 = 161.47, p < 0.001; Fig. 2A). Although there appeared to have been a reduction in nesting success (the proportion of eggs laid that hatched and survived to fledge), from 63% in 1962 (Vermeer 1963, in Hayward and Verbeek 2020) to a mean of 52% for comparable surveys conducted 2008-2010 (2008: 0.43; 2009: 0.50; 2010: 0.56; Fig. 2B), the decline was not statistically significant (F1,2 = 2.734, p = 0.24).
DISCUSSION
We compared historical and contemporary breeding records from a colony within a population of Glaucous-winged Gulls that has experienced significant population declines in past decades, following an increasing trend from about 1900 to the mid-1980s (Blight et al. 2015a). Although previous work has documented long-term declines in reproductive investment for this species in the Salish Sea in the form of declining egg and clutch size (Blight 2011), this current study examined reproductive outcomes. We showed that present-day breeders on average experienced lower hatching success and may have also produced fewer chicks than birds breeding historically (1960s to 1980s) on Mandarte Island, one of the largest Glaucous-winged Gull colonies in the region.
In contrast, our results indicate that within-season patterns of reproductive investment at this colony are at least partially similar to those described in previous studies. Both Vermeer (1963) and Verbeek (1986) recorded an intra-annual decline in clutch size with lay date while assessing “early” vs. “late” Glaucous-winged Gull clutches at Mandarte Island (though the negative within-season clutch size trend documented by Vermeer was non-significant). In the same study, Verbeek also assessed egg size over the season and found that it remained constant. Our results showed that females breeding later in the season have continued to produce smaller clutches than those laying earlier in the season, and they now also produce smaller eggs.
Current seasonal patterns of reproductive investment at the Glaucous-winged Gull colony on Mandarte Island are thus partially reflective of those recorded at the colony in the 1960s to 1980s, but with an important difference: later layers now lay smaller eggs on average, as well as fewer of them. Egg and clutch size in gulls are strongly linked to diet, particularly in food-limited populations (e.g., Hiom et al. 1991, Bolton et al. 1993). Despite historical declines in availability and perhaps quality of forage fish populations in the Salish Sea (Wallace 1998, Therriault et al. 2009, Boldt et al. 2021), and long-term dietary changes in Glaucous-winged Gulls (Blight et al. 2015b), some individuals (i.e., higher-quality females) may still retain access to this important source of nutrients pre-laying. These higher-quality individuals are still able to lay earlier and larger clutches (early-season mean clutch size is approximately three eggs) than poorer-quality females, who may be more reliant on substandard quality and/or quantity of food in the pre-laying period (e.g., Annett and Pierotti 1999). Some slight support for this individual-quality argument is provided by the fact that our study population produced one supra-normal clutch of four eggs (which failed pre-hatch) and one supra-normal brood of four chicks (reared by a pair that had originally hatched three eggs). The fourth chick appeared at this nest partway through the chick rearing period and was regularly observed being fed by the parents, with all four chicks successfully reared to fledge. Over the course of our broader three-year study of the Salish Sea population, we also recorded two additional four-egg clutches at nearby colonies (Great Chain Island, 2009; White Islets, 2010), though all eggs were large, suggesting the possibility of female-female pairs.
This pattern in reproductive investment of an intra-seasonal clutch size decline has been reported in other Larid species (e.g., Sydeman et al. 1991, Bolton et al. 1993) in which three-egg clutches are typically laid by females who reach breeding condition earlier, while one- or two-egg clutches are laid later in the season by poorer-quality females. For Herring Gulls (L. argentatus), Kilpi et al. (1996) suggested that egg-size variation within three-egg clutches increases as food availability decreases until a threshold level when two-egg clutches become more frequent. Indeed, a comparative study of California Gulls (L. californicus) breeding in a high and low food environment found a modal clutch size of two at a colony where pre-laying food availability was low, and a modal clutch size of three at a nearby colony with greater food availability in the pre-laying period (Winkler 1985). It has been shown that females laying clutches of two eggs are capable of laying replacement eggs if food induced (Winkler 1985, Reid 1988), and thus the degree to which plasticity in response to environmental conditions may also contribute to declines in clutch size with lay date as a means of conserving future reproductive potential are unclear (Kilpi et al. 1996).
Chick mass at hatch did not decline with seasonal progression. Egg mass typically correlates with hatching mass of chicks in gulls (Kilpi et al. 1996), and thus our finding that egg mass declined with lay date (even when controlling for other reproductive parameters) is surprising. It may be that although later-laid eggs were lighter, critical nutrients for chick development were present in sufficient quantities to promote growth. Alternatively, this result may be because of lower survival of the smallest eggs laid later in the season, with only larger late-laid embryos surviving through to hatch, i.e., pre-hatch failure of the smallest eggs in the sample. This explanation is consistent with other studies showing that egg size affects hatchability (Martin 1987).
We found only limited evidence of a significant seasonal decline in the number of chicks fledged per nest. It must be noted, however, that in 2008 and 2010 our fledging success data collection schedule excluded nests still active late in the season, when fledging success is generally lower (Borboroglu et al. 2008). Including the latest breeders may well have shown a greater seasonal decline in fledging success, as it did in 2009, when the negative trend was driven by the very late nests, something that has been observed in studies of other gulls (Sydeman et al. 1991).
Previous results have indicated that overall reproductive investment has declined for Glaucous-winged Gulls in the Salish Sea over the past century (Blight 2011), and our study suggests a similar long-term trend in reproductive outcomes from the 1960s, 1970s, and 1980s, supporting the suggestion that declines in reproductive output over time have been sufficient to drive the population decline that commenced around the mid-1980s (Blight et al. 2015a). A comparison of hatch success recorded in the 1960s to 1980s to data collected in the twenty-first century indicates that hatch success appears to have declined over time from approximately 83% to 60%. The causes of this decline are unclear. Egg size has decreased in this population over time, a factor that may negatively influence hatch success if it leads to egg nutritional content being insufficient to support chick growth (Martin 1987). Alternatively, or additionally, environmental contaminants may have contributed to greater egg failure in the twenty-first century. Although levels of legacy contaminants such as PCBs and DDT/DDE have gradually declined in the North Pacific marine environment over time (Elliott and Elliott 2013), environmental levels of polybrominated diphenyl ethers (PBDEs) increased from the 1990s to the early 2000s, particularly in areas near urban environments such as the Salish Sea. These have since decreased in response to phase-outs, but concentrations of non-PBDE flame retardants have continued to increase (Chen et al. 2012, Miller et al. 2014, 2015). Detrimental effects of these PBDE and non-PBDE flame retardants have been demonstrated in lab studies (e.g., Crump et al. 2010). However, legacy persistent organochlorine pollutants such as DDT did not decline in coastal environments until the 1980s (Elliott and Elliott 2013), meaning the region’s gulls studied historically, and serving as our points of comparison, were also exposed to endocrine-disrupting contaminants. They have likely also been exposed to increasing amounts of methyl mercury over time, but this exposure has been ameliorated to some degree by shifts to lower trophic-level diets on average (Choy et al. 2022).
Despite reduced hatch success in contemporary records compared to historical values, we did not find a significant decline in the proportion of eggs laid that survived to fledge. Although nesting success did decline from 63% in 1962 to a mean of 52% for 2008-2010, the trend was non-significant. This analysis is reliant on a single historical value; thus, it is difficult to draw strong conclusions from the result.
Overall, we identified noteworthy trends in modern vs. historical reproductive parameters in this quasi-urban gull population. Reproductive outcomes (hatch success) were significantly lower compared to historic records when the population was at, or approaching, its historical maximum size (Blight et al. 2015a). Despite a decrease in population size presumably reducing density-dependent effects, at least one aspect of the modern intra-seasonal reproductive patterns — clutch size in relation to lay date — was similar to historical trends. Our results comparing historical and contemporary breeding outcomes indicated lower breeding success, speaking to downstream effects of decreased egg and clutch sizes, and the potential for continued population impacts of environmental stressors such as reduced availability and/or quality of fish prey. Although declining and then recovering populations of Bald Eagles (Haliaeetus leucocephalus; Hipfner et al. 2012) have likely also contributed to recent Glaucous-winged Gull declines in the broader Salish Sea (Blight et al. 2015a, Henson et al. 2019), there was only limited evidence for an impact of eagle predation on our Mandarte Island study population specifically. During our modern data collection period, the gull colony at Mandarte Island only experienced low levels of eagle predation, perhaps due to the documented presence on a nearby island of a territorial pair, so that other individuals were excluded from the area (cf. Hipfner et al. 2011; L. K. Blight, unpublished data). This is in contrast to the similarly sized colony on Mitlenatch Island at the north end of the Salish Sea, where immature Bald Eagles seem to take high numbers of gull eggs (L. K. Blight, personal observation). In other words, eagle effects may be colony specific. However, given the very limited number of years covered by our historical data, we cannot rule out longer-term eagle effects at Mandarte Island.
Despite a tendency toward reduced reproductive outcomes, some individual females (pairs) continue to do well in the modern colony, fledging full broods of three chicks. In a study of this same species in Alaska, Murphy et al. (1992) showed that inter-pair differences in reproductive performance were accentuated under detrimental conditions, so that in good years, most birds did well regardless of individual quality, whereas in poor years, a subset of the pairs that did well in good years — the high-quality birds — continued to do just as well. It appears that this same issue of individual quality may be at play in our system, albeit over several decades, because some individuals continue to produce three eggs and, with their mate, rear them through to fledging, despite the fact that the population is on average producing fewer young than it did historically.
Anthropogenic change to the marine environment is threatening bird populations worldwide (Dias et al. 2019). Climate change, overfishing, and pollution all contribute to declines in food availability, which has negative consequences for marine bird reproductive success and ultimately population health (Dias et al. 2019). Recently, interesting developments in the ecology of fear have suggested that response to predators may drive reproductive outcomes in passerine birds (Zanette et al. 2011, Allen et al. 2022). Whether such a mechanism may be contributing to changing reproductive outcomes in gulls (e.g., through increased vigilance at the expense of foraging) is perhaps also a topic worthy of further study. For Glaucous-winged Gull management in the Salish Sea, further identifying the drivers of long-term declines in reproductive outcomes will be key to managing the species in this area. Although we reviewed several historical papers from our study site, we found few datasets we could use for comparison. Long-term studies are essential for revealing how anthropogenic changes influence population dynamics and the mechanisms underlying such changes (Wooler et al. 1992), but methodological clarity and correct reporting are essential to ensure data are comparable through time.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
Field research (2008-2010) was carried out under Permit No. A07-0309 from the University of British Columbia's Animal Care Committee and under Research Permit No. BC-10-0057 from the Canadian Wildlife Service. Collection of author-generated field data was carried out using protocols designed to minimize disturbance to breeding birds. LKB was funded by grants from the National Sciences and Engineering Research Council of Canada, the Koerner Foundation, the Canadian Federation of University Women (Dr. Alice E. Wilson Award), the American Museum of Natural History (Lerner-Gray Grant for Marine Research), the Waterbird Society (Nisbet Grant), and a Hesse Research Award from the University of British Columbia. Work by LKB, WOS, and GM was supported by cumulative effects funding from Environment and Climate Change Canada. Comments by three anonymous reviewers improved the manuscript.
LITERATURE CITED
Allen, M. C., M. Clinchy, and L. Y. Zanette. 2022. Fear of predators in free-living wildlife reduces population growth over generations. Proceedings of the National Academy of Sciences 119(7):e2112404119. https://doi.org/10.1073/pnas.2112404119
Annett C. A., and R. Pierotti. 1999. Long-term reproductive output in Western Gulls: consequences of alternate tactics in diet choice. Ecology 80(1):288-297. https://doi.org/10.1890/0012-9658(1999)080[0288:LTROIW]2.0.CO;2
Blight, L. K. 2011. Egg production in a coastal seabird, the Glaucous-winged Gull, Larus glaucescens, declines during the last century. PLOS One 6(7):e22027. https://doi.org/10.1371/journal.pone.0022027
Blight, L. K. 2014. Glaucous-winged Gull (Larus glaucescens) colony count data, Georgia Basin, BC, Canada, 1900-2010. Figshare. Dataset. https://doi.org/10.6084/m9.figshare.1218437.v1
Blight, L. K., D. F. Bertram, and E. Kroc. 2019. Evaluating UAV-based techniques to census an urban-nesting gull population on Canada’s Pacific coast. Journal of Unmanned Vehicle Systems 7(4):312-324. https://doi.org/10.1139/juvs-2019-0005
Blight, L. K., M. C. Drever, and P. Arcese. 2015a. A century of change in Glaucous-winged Gull, Larus glaucescens populations in a dynamic coastal environment. Condor 117(1):108-120. https://doi.org/10.1650/CONDOR-14-113.1
Blight, L. K., K. A. Hobson, T. K. Kyser, and P. Arcese. 2015b. Changing gull diet in a changing world: a 150-year stable isotope (δ13C, δ15N) record from feathers collected in the Pacific Northwest of North America. Global Change Biology 21(4):1497-1507. https://doi.org/10.1111/gcb.12796
Boldt, J.L., A. Javorski, and P.C. Chandler. 2021. State of the physical, biological, and selected fishery resources of Pacific Canadian marine ecosystems in 2020. Canadian Technical Report of Fisheries and Aquatic Sciences, Fisheries and Oceans Canada, Institute of Ocean Sciences, Sidney, British Columbia, Canada. ”_blank”"> https://publications.gc.ca/collections/collection_2021/mpo-dfo/Fs97-6-3434-eng.pdf
Bolton, M. 1991. Determinants of chick survival in the Lesser Black-backed Gull: relative contributions of egg size and parental quality. Journal of Animal Ecology 60(3):949-960. https://doi.org/10.2307/5424
Bolton, M., P. Monaghan, and D. C. Houston. 1993. Proximate determination of clutch size in Lesser Black-backed Gulls: the roles of food supply and body condition. Canadian Journal of Zoology 71(2):273-279. https://doi.org/10.1139/z93-039
Borboroglu, G., P. Yorio, P. Moreno, and J. Potti. 2008. Seasonal declines in breeding performance of the Kelp Gull, Larus dominicanus. Marine Ornithology 36(2):153-157. http://www.marineornithology.org/content/get.cgi?rn=783
Chastel, O., H. Weimerskirch, and P. Jouventin. 1995. Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76(7):2240-2246. https://doi.org/10.2307/1941698
Chen, D., R. J. Letcher, N. M. Burgess, L. Champoux, J. E. Elliott, C. E. Hebert, P. Martin, M. Wayland, D. C. Weseloh, and L. Wilson. 2012. Flame retardants in eggs of four gull species, Laridae, from breeding sites spanning Atlantic to Pacific Canada. Environmental Pollution 168:1-9. https://doi.org/10.1016/j.envpol.2012.03.040
Choy, E.S., L.K. Blight, J.E. Elliott, K.H. Hobson, M. Zanuttig, and K. H. Elliott. 2022. Stable mercury trends support a long-term diet shift away from marine foraging in Salish Sea Glaucous-winged Gulls over the last century. Environmental Science and Technology 56:12097–12105. ”_blank”"> https://doi.org/10.1021/acs.est.1c03760
Crump, D., C. Egloff, S. Chiu, R. J. Letcher, S. Chu, and S. W. Kennedy. 2010. Pipping success, isomer-specific accumulation, and hepatic mRNA expression in chicken embryos exposed to HBCD. Toxicological Sciences 115(2):492-500. https://doi.org/10.1093/toxsci/kfq068
Cury, P. M., I. L. Boyd, S. Bonhommeau, T. Anker-Nilssen, R. J. M. Crawford, R. W. Furness, J. A. Mills, E. J. Murphy, H. Österblom, M. Paleczny, J. F. Piatt, J.-P. Roux, L. Shannon, and W. J. Sydeman. 2011. Global seabird response to forage fish depletion-one-third for the birds. Science 334(6063):1703-1706. https://doi.org/10.1126/science.1212928
Cushing, D. H. 1990. Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Advances in Marine Biology 26:249-293. https://doi.org/10.1016/S0065-2881(08)60202-3.
Dias, M. P., R. Martin, E. J. Pearmain, I. J. Burfield, C. Small, R. A. Phillips, O. Yates, B. Lascelles, P. G. Borboroglu, and J. P. Croxall. 2019. Threats to seabirds: a global assessment. Biological Conservation 237:525-537. https://doi.org/10.1016/j.biocon.2019.06.033
Davis, M. L., J. E. Elliott, and T. D. Williams. 2015. Spatial and temporal variation in the dietary ecology of the Glaucous-winged Gull, Larus glaucescens, in the Pacific Northwest. Marine Ornithology 43(2):189-198. http://www.marineornithology.org/content/get.cgi?rn=1130
Elliott, J. E., and K. H. Elliott. 2013. Tracking marine pollution. Science 340(6132):556-558. https://doi.org/10.1126/science.1235197
Grant, M. C. 1991. Relationships between egg size, chick size at hatching, and chick survival in the Whimbrel, Numenius phaeopus. Ibis 133(2):127-133. https://doi.org/10.1111/j.1474-919X.1991.tb04823.x
Hayward, J. L., and N. A. M. Verbeek. 2020. Glaucous-winged Gull, Larus glaucescens, version 1.0. In S. M. Billerman, editor. Birds of the World. Cornell Lab of Ornithology, Ithaca, New York, USA. https://doi.org/10.2173/bow.glwgul.01
Hebert, C. E., J. L. Shutt, and R. O. Ball. 2002. Plasma amino acid concentrations as an indicator of protein availability to breeding Herring Gulls, Larus argentatus. Auk 119(1):185-200. https://doi.org/10.1093/auk/119.1.185
Henson, S. M., R. A. Desharnais, E. T. Funasaki, J. G. Galusha, J. W. Watson, and J. L. Hayward. 2019. Predator-prey dynamics of Bald Eagles and Glaucous-winged Gulls at Protection Island, Washington, USA. Ecology and Evolution 9(7):3850-3867. https://doi.org/10.1002/ece3.5011
Hipfner, J. M., L. K. Blight, R. W. Lowe, S. I. Wilhelm, G. J. Robertson, R. T. Barrett, T. Anker-Nilssen, and T. P. Good. 2012. Unintended consequences: how the recovery of Sea Eagle, Haliaeetus, spp. populations in the northern hemisphere is affecting seabirds. Marine Ornithology 40(1):39-52. http://www.marineornithology.org/PDF/40_1/40_1_39-52.pdf
Hipfner, J.M., K. W. Morrison, and R. Darvill. 2011. Peregrine Falcons enable two species of colonial seabirds to breed successfully by excluding other aerial predators. Waterbirds 34(1):82-88. https://doi.org/10.1675/063.034.0110
Hiom, L., M. Bolton, P. Monaghan, and D. Worrall. 1991. Experimental evidence for food limitation egg production in gulls. Ornis Scandinavica 22(2):94-97. https://doi.org/10.2307/3676539
Hobson, K. A., L. K. Blight, and P. Arcese. 2015. Human-induced long-term shifts in gull diet from marine to terrestrial sources in North America’s coastal Pacific: more evidence from more isotopes (δ2H, δ34S). Environmental Science and Technology 49(18):10834-10840. https://doi.org/10.1021/acs.est.5b02053
Hooper, T. D., 1988. Habitat, reproductive parameters, and nest-site tenacity of urban-nesting Glaucous-winged Gulls at Victoria, British Columbia. Murrelet 69(1):10-14. https://doi.org/10.2307/3534880
Houston, D. C., P. J. Jones, and R. M. Sinly. 1983. The effect of female body condition on egg laying in Lesser Black-backed Gulls, Larus fuscus. Journal of Zoology 200(4):509-520. https://doi.org/10.1111/j.1469-7998.1983.tb02812.x
Hunt, Jr., G. L., and M. W. Hunt. 1976. Gull chick survival: the significance of growth rates, timing of breeding and territory size. Ecology 57(1):62-75. https://doi.org/10.2307/1936398
Kilpi, M., L. Hillstrom, and K. A. I. Lindstrom. 1996. Egg-size variation and reproductive success in the Herring Gull, Larus argentatus: adaptive or constrained size of the last egg? Ibis 138(2):212-217. https://doi.org/10.1111/j.1474-919X.1996.tb04330.x
Martin, T. E. 1987. Food as a limit on breeding birds: a life-history perspective. Annual Review of Ecology and Systematics 18(1):453-487. https://doi.org/10.1146/annurev.es.18.110187.002321
McKechnie, I., D. Lepofski, M. L. Moss, V. L. Butler, T. J. Orchard, G. Coupland, F. Foster, M. Caldwell, and K. Lertzman. 2014. Archaeological data provide alternative hypotheses on Pacific herring, Clupea pallasii, distribution, abundance, and variability. Proceedings of the National Academy of Sciences 111(9):E807-E816. https://doi.org/10.1073/pnas.1316072111
Miller, A., J. E. Elliott, K. H. Elliott, M. F. Guigueno, L. K. Wilson, S. Lee, and A. Idrissi. 2014. Spatial and temporal trends in brominated flame retardants in seabirds from the Pacific coast of Canada. Environmental Pollution 195:48-55. https://doi.org/10.1016/j.envpol.2014.08.009
Miller, A., J. E. Elliott, K. H. Elliott, M. F. Guigueno, L. K. Wilson, S. Lee, and A. Idrissi. 2015. Brominated flame-retardant trends in aquatic birds from the Salish Sea region of the west coast of North America, including a mini-review of recent trends in marine and estuarine birds. Science of the Total Environment 502:60-69. https://doi.org/10.1016/j.scitotenv.2014.09.006
Mills, J. A., J. W. Yarrall, J. M. Bradford-Grieve, M. J. Uddstrom, J. A. Renwick, and J. Merilä. 2008. The impact of climate fluctuation on food availability and reproductive performance of the planktivorous Red-billed Gull, Larus novaehollandiae scopulinus. Journal of Animal Ecology 77(6):1129-1142. https://doi.org/10.1111/j.1365-2656.2008.01383.x
Murphy, E. C., A. A. Hoover-Miller, R. H. Day, and K. L. Oakley. 1992. Intracolony variability during periods of poor reproductive performance at a Glaucous-winged Gull colony. Condor 94(3):598-607. https://doi.org/10.2307/1369245
Parsons, J. 1970. Relationship between egg size and post-hatching chick mortality in the Herring Gull, Larus argentatus. Nature 228(5277):1221-1222. https://doi.org/10.1038/2281221a0
R Core Team. 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Real, E., D. Oro, A. Martinez-Abrain, J. M. Igual, A. Bertolero, M. Bosch, and G. Tavecchia. 2017. Predictable anthropogenic food subsidies, density-dependence, and socio-economic factors influence breeding investment in a generalist seabird. Journal of Avian Biology 48(11):1462-1470. https://doi.org/10.1111/jav.01454
Reid, W. V. 1987a. The cost of reproduction in the Glaucous-winged Gull. Oecologia 74(3):458-467. https://doi.org/10.1007/BF00378945
Reid, W. V. 1987b. Constraints on clutch size in the Glaucous-winged Gull. Studies in Avian Biology 10:8-25. https://sora.unm.edu/node/139218
Reid, W. V. 1988. Population dynamics of the Glaucous-winged Gull. Journal of Wildlife Management 52(4):763-770. https://doi.org/10.2307/3800944
Ricklefs, R. E. 1973. Fecundity, mortality, and avian demography. Pages 366-435 in D. S. Farner, editor. Breeding biology of birds: proceedings of a symposium on breeding behavior and reproductive physiology in birds. National Research Council, The National Academies Press, Washington, D.C., USA. https://nap.nationalacademies.org/read/20406/chapter/10
Stearns, S. C. 1976. Life-history tactics: a review of the ideas. Quarterly Review of Biology 51(1):3-47. https://doi.org/10.1086/409052
Stearns, S. C. 1992. The evolution of life histories. Oxford University Press, New York, New York, USA.
Sydeman, W. J., J. F. Penniman, T. M. Penniman, P. Pyle, and D. G. Ainley. 1991. Breeding performance in the Western Gull: effects of parental age, timing of breeding and year in relation to food availability. Journal of Animal Ecology 60(1):135-149. https://doi.org/10.2307/5450
Therriault, T. W., D. E. Hay, and J. F. Schweigert. 2009. Biological overview and trends in pelagic forage fish abundance in the Salish Sea, (Strait of Georgia, British Columbia). Marine Ornithology 37:3-8. http://www.marineornithology.org/content/get.cgi?rn=805
Verbeek, N. A. M. 1986. Aspects of the breeding biology of an expanded population of Glaucous-winged Gulls in British Columbia. Journal of Field Ornithology 57(1):22-33. https://sora.unm.edu/node/51235
Verbeek, N. A. M, and H. Richardson. 1982. Limits to egg size in gulls: another point of view. Journal of Field Ornithology 53(2):168-170.https://sora.unm.edu/node/50950
Vermeer, K. 1963. The breeding biology of the Glaucous-winged Gull (Larus glaucescens) on Mandarte Island, B.C. Thesis. University of British Columbia, Vancouver, Canada.https://dx.doi.org/10.14288/1.0105376
Vermeer, K. 1992. Population growth of the Glaucous-winged Gull, Larus glaucescens, in the Strait of Georgia, British Columbia, Canada. Ardea 80(1):181-185.
Vermeer K., and K. Devito. 1989. Population trends of nesting Glaucous-winged Gulls in the Strait of Georgia. Pages 88-93 in K. Vermeer and R. W. Butler, editors. The ecology and status of marine and shoreline birds in the Strait of Georgia, British Columbia. Canadian Wildlife Service Special Publication, Ottawa, Ontario, Canada.
Vermeer, K., D. Power, and G. J. Smith. 1988. Habitat selection and nesting biology of roof-nesting Glaucous-winged Gulls. Colonial Waterbirds 11(2):189-201. https://doi.org/10.2307/1521000
Wallace, S. S. 1998. Changes in human exploitation of marine resources in British Columbia (pre-contact to present day). Pages 56-62 in D. Pauly, T. J. Pitcher, D. Preikshot, and J. Hearne, editors. Back to the future: reconstructing the Strait of Georgia's ecosystem. Fisheries Centre Research Reports 6. Fisheries Centre, University of British Columbia, Vancouver, Canada. ”_blank”">http://dx.doi.org/10.14288/1.0348125
Ward, J. G. 1973. Reproductive success, food supply, and the evolution of clutch-size in the Glaucous-winged Gull. Dissertation. University of British Columbia, Vancouver, Canada. https://dx.doi.org/10.14288/1.0101109
Wickham, H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, New York, USA.
Winkler, D. W. 1985. Factors determining a clutch size reduction in California Gulls, Larus californicus: a multi-hypothesis approach. Evolution 39(3):667-677. https://doi.org/10.2307/2408661
Wooler, R. D., J. S. Bradley, and J. P. Croxall. 1992. Long-term population studies of seabirds. Trends in Ecology and Evolution 7(4):111-114. https://doi.org/10.1016/0169-5347(92)90143-Y
Zanette, L. Y., A. F. White, M. C. Allen, and M. Clinchy. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334(6061):1398-1401. http://doi.org/10.1126/science.1210908
Fig. 1

Figure 1. Intra-annual trends in reproductive investment and outcomes for Glaucous-winged Gulls (Larus glaucescens) at Mandarte Island, Canada. (A) Plot of mean daily clutch size vs. lay date (day of year) combining data from 2008, 2009, and 2010. (B) Plot of mean egg mass (g) vs. lay date combining data from 2008, 2009, and 2010. (C) Plot of mean daily chick mass (g) at hatch vs. lay date combining data from 2008, 2009, and 2010. (D) Mean daily fledging success vs. lay date combining data from 2008, 2009, and 2010. To reduce visual clutter, plotted points are daily means; however, best fit curves are fit to the raw disaggregated data.

Fig. 2

Figure 2. Long-term trends in Glaucous-winged Gull (Larus glaucescens) reproductive outcomes at Mandarte Island, Canada. (A) Hatch success (number of chicks hatched per number of eggs laid) vs. year. Data sources: Vermeer (1963), Verbeek (1986), this study (2008-2010). (B) Nest success (proportion of eggs laid that fledged chicks) vs. year. Data sources: Vermeer (1963) in Hayward and Verbeek (2020), this study (2008-2010).