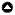The following is the established format for referencing this article:
Rossignaud, L., D. Kelly, E. B. Spurr, D. J. Flaspohler, R. B. Allen, and E. G. Brockerhoff. 2022. Trends in bird counts 1978–2020 in a New Zealand Nothofagus forest with variable control of mammalian predators. Avian Conservation and Ecology 17(2):4.ABSTRACT
Many New Zealand native bird species are threatened by introduced mammalian predators, and pest management programs are common in the country. Despite that, measuring the efficacy of such programs is often limited by resources, and thus the long-term population status of many native birds is not well documented. Here, we examined long-term population trends of forest bird species and changes in the bird community structure at Craigieburn Forest Park, where there was intermittent control of stoats (Mustela erminea). We analyzed 10,938 5-min bird point counts covering the periods 1978–1982, 1999–2004, and 2019–2020 in an old-growth Nothofagus (southern beech) forest. We assessed trends over time in the counts of each bird species with season, elevation, and site as co-variables. We also tested for a relationship with variable seed crops of the mast-seeding canopy tree, N. solandri var. cliffortioides. Bellbird (Anthornis melanura) was the only native species showing a continuous increase over time. In the first 25 years of the study, stoat control was intermittent, and more native birds decreased than increased. In later years, stoat control was continuous, and more native species increased than decreased. Large Nothofagus seed crops were associated with significant increases in all six exotic bird species tested, but only one of nine native bird species. These findings suggest that long-term trends of bird populations are influenced by the interactions of species vulnerability to stoat predation and the consistency of pest control efforts. Unfortunately, ship rats (Rattus rattus), which were absent at Craigieburn before 2010, are now common and may pose a new threat to native birds. Our results show that systematic long-term bird and seedfall monitoring, including careful archiving of sampling information, is helpful to guide conservation of the remaining native birds of New Zealand.RÉSUMÉ
INTRODUCTION
There is concern worldwide about bird population declines, including some common species (Bowler et al. 2019, Rosenberg et al. 2019). New Zealand’s biodiversity has experienced major declines and extinction events (e.g., Şekercioğlu et al. 2004, Kelly and Sullivan 2010) resulting from direct and indirect impacts of human colonization in about 1280 A.D. (Wilmshurst et al. 2008, Innes et al. 2010). Birds were particularly affected, with about 59 species going extinct and 71 of the remaining native bird species being threatened, including 25 that are critically endangered (Robertson et al. 2017). Such species loss can strongly impact ecosystem processes and services by reducing bird species density (Anderson et al. 2011, Iles and Kelly 2014). Many endemic species that persist are now functionally extinct or range restricted (e.g., Stitchbird, Notiomystis cinta, Kelly et al. 2006, Walker and Monks 2018). The dominant role of introduced mammalian predators as a cause of decline of native birds in New Zealand has been demonstrated clearly (Innes et al. 2010). Despite recent publications on general long-term bird population trends using bird species distributions (Walker and Monks 2018, Walker et al. 2019) or meta-analysis (Fea et al. 2020), detailed trend data for native bird populations for periods longer than 10 yr are rare. This is especially true for species that appear to remain common, and limits the ability to detect gradual population declines locally and nationally (Elliott et al. 2010, Miskelly 2018).
Together with human-caused habitat change, predation by introduced mammalian predators such as rats (especially Rattus rattus), stoats (Mustela erminea), brushtail possums (Trichosurus vulpecula), weasels (Mustela nivalis), and feral cats (Felis catus) contributed to the rapid extinction of many New Zealand endemic birds (Holdaway 1989, 1999) and remains the main threat to surviving endemic and native bird species (Innes et al. 2010). There could also be impacts from competition for food by mice (Mus musculus), rats, and introduced wasps (Vespula spp.) (Beggs 2001, Innes et al. 2010). Introduced mammals and wasps are well established in much of New Zealand and their interactions with native species are complex and challenge pest control efforts. For example, Nothofagus species (southern beech), which dominate forests in cooler parts of New Zealand, show mast seeding, i.e., highly variable, synchronous seed crops among years (King 1983, Allen and Platt 1990, Schauber et al. 2002, Smaill et al. 2011, Kelly et al. 2013). Mouse and rat populations increase after Nothofagus mast seed crops, followed by increases of their chief predator, stoats (King 1983, Alley et al. 2001, Harris et al. 2022). These predator irruptions then cause reductions in native birds (Kelly et al. 2005, 2008).
National and regional government agencies have pursued campaigns of intensive pest control for many decades with the goal of preserving endangered endemic bird species (Russell et al. 2015). Ongoing pest control programs of various intensity are conducted across the main islands with small-scale control of rats, stoats, possums (Miskelly and Robertson 2002, Kelly et al. 2005, Harris et al. 2022), and wasps (Lester et al. 2013) and larger-scale aerial application of 1080 poison (O'Donnell and Hoare 2012, Elliott and Kemp 2016). Several shorter-term (<10 yr) studies have demonstrated that targeted pest control efforts can suppress pest numbers and even allow threatened bird populations to recover (Graham and Veitch 2002, Kelly et al. 2005). Importantly, some species that are still extant and were believed to have stable populations, were found to be gradually declining, including Mohua (Mohoua ochrocephala), Kaka (Nestor meridionalis), and Kea (Nestor notabilis) (Elliott et al. 1996, Moorhouse et al. 2003, Robertson et al. 2017). A similar phenomenon has been seen outside New Zealand in continental bird populations, which have experienced dramatic declines in recent decades, including in North America (Rosenberg et al. 2019) and Europe (Bowler et al. 2019). This suggests that conservation efforts may need to be invested in maintaining not only known rare and endangered birds but also still-widespread seemingly common endemic species (Elliott et al. 2010).
Although few long-term New Zealand studies exist, there is evidence that declines of non-threatened bird species are continuing (Innes et al. 2010, Walker and Monks 2018). For instance, the New Zealand Bird Atlas data documenting bird species distributions revealed declining ranges 1985–2007 for 25 of 66 endemic bird taxa, including some that were still widespread, such as the Rifleman (Acanthisitta chloris), Brown Creeper (Mohoua novaeseelandiae), and Tomtit (Petroica macrocephala) (Robertson et al. 2007: Append. K). Although such range reductions are suggestive of population declines, little long-term abundance data are available for these relatively common species (Innes et al. 2010). A study by Elliott et al. (2010) in the northern South Island investigated long-term bird population trends in an area of Nelson Lakes National Park with no pest control programs. There, they showed that the native bird community changed significantly between 1974 and 2007, with declines in several common bird species such as Bellbird (Anthornis melanura), Rifleman, and Tomtit. They speculated that a growing brushtail possum population and the arrival of Vespula wasps were probable causes of these declines. Such short- and long-term studies highlight the potential diversity of responses observed among different bird species as well as the general lack of information about relatively common bird species that could be experiencing undetected declines under current levels of pest control (Elliott et al. 2010). We suggest that this is an important question for the future of New Zealand’s native bird populations and for emerging initiatives such as the Predator-Free New Zealand 2050 campaign (Owens 2017).
Here, we use bird count data to examine long-term bird population trends (1978–2020) in Craigieburn Forest Park, an old-growth mountain beech forest (Nothofagus solandri var. cliffortioides, synonym Fuscospora cliffortioides), which has had intermittent pest control (King 1983, Kelly et al. 2005, Harris et al. 2022). Of the eight most common native birds at Craigieburn in 1999–2000 (Murphy and Kelly 2003), four were listed by Robertson et al. (2007) as decreasing in range nationally (Rifleman, Brown Creeper, Tomtit, Kea). We wanted to measure trends in an area with some pest control, to compare with those reported for unmanaged Nothofagus forest in the northern South Island by Elliott et al. (2010). Specifically, our aims were to (1) measure year-to-year changes in forest bird populations at Craigieburn using 13 yr of point counts over three studies between 1978 and 2020; (2) examine associations of bird counts with mountain beech seedfall; and (3) test for long-term changes in bird counts over the three studies across the 43-yr timespan. We discuss these findings in relation to pest control programs at Craigieburn and the potential impact of ship rat arrival after 2010. We included both common native and exotic bird species in our analyses because exotic species can display different responses to biotic events and pest control (O'Donnell and Hoare 2012, Miskelly 2018).
METHODS
Studies Used in Analyses
We analyzed data from three studies carried out in Craigieburn Forest Park over a span of 43 yr. The first study from 1978 to 1982 was led by EBS and represents the largest unpublished set of point counts for New Zealand forest birds (Hartley 2012). The second study from 1999 to 2004 was partially published by Murphy and Kelly (2003) and Kelly et al. (2005). The third study was in 2019 and 2020, led by LR, DF, and DK, and is published here. All bird counts were point counts performed following the standard 5-min bird count method in widespread use in New Zealand (Dawson and Bull 1975). Differences among studies in the exact location of count stations, the time of year of counts, and observers involved were corrected for in the analysis (see below).
Study Area
The bird surveys took place in Craigieburn Forest Park, Canterbury, New Zealand (centered around 171° 42.5’ E, 43° 91.1’ S) (Fig. 1), which is managed by the New Zealand Department of Conservation. The counts were all in old-growth native mountain beech forest, which is bounded at lower elevation by cleared pastoral lands and exotic conifer (Pinus spp. and Pseudotsuga menziesii) plantations, and at higher elevation by undisturbed snow tussock (Chionochloa spp.) alpine grassland. The forest canopy is almost pure mountain beech, which shows strong mast seeding (Allen and Platt 1990). The whole study area of about 1,000 ha is contiguous forest with similar montane climate, vegetation, and aspect (Shanks et al. 1990). Although the climate is cool-temperate with occasional snow below the treeline in winter, almost all the avifauna is resident year round. Only one migratory bird (the Long-tailed Cuckoo (Eudynamys taitensis)) is present.
Bird Counts
Counts were performed in three adjoining catchments (Broken River, Cheeseman, and Craigieburn), hereafter called sites. Most of the 10,938 counts were in Broken River (88%), with 9% in Cheeseman, and 3% in Craigieburn. In the first study, between 1978 and 1982, 8,320 bird counts were conducted, all in Broken River (Table 1, Fig. 1). Counts were done along four tracks (subsites) in April and October 1978, April, May, June, August, and October 1979, and every 2 mo from February 1980 to December 1982. The second study was carried out between May 1999 and January 2004 (Kelly et al. 2005), and birds were counted at Broken River, Cheeseman, and Craigieburn (Table 1, Fig. 1). Bird counts were done in May and September 1999, October 2001, and every January from 2000 to 2004. The third study was performed in March–June 2019 and January 2020 on three of the four Broken River subsites plus Cheeseman and Craigieburn. All species (except Long-tailed Cuckoo) were resident all year, but the conspicuousness (detectability) of each species is known to vary seasonally (Dawson et al. 1978). Our intensive sampling every 2 mo in 1980–1982 allowed us to correct for seasonal changes in detectability in the analysis.
Bird count stations were spaced 200 m apart along each of the gravel roads or walking tracks, ranging from 800 m elevation to the tree line at about 1,300 m. At each marked station, experienced counters recorded all birds seen or heard for 5 min (Dawson and Bull 1975). Counts in the first study were unbounded, but in the second and third studies were restricted to an estimated 100 m radius, but this should make little difference. The maximum detection range for most species in Craigieburn Forest Park was much less than 100 m, so the 100 m radius (when applied) only excluded a few distant records of species with loud calls (mainly Bellbird) on calm days. Bird counts only took place in favorable weather (no rain, low wind), typically between 9 a.m. and 4 p.m. New Zealand forest point counts typically run through the day, excluding dawn and dusk when calling rates are higher (Dawson et al. 1978, Elliott et al. 2010). Variation in detection rates during the day is modest, and sampling was balanced across morning and afternoon.
Variation among observers was minimized in several ways. Most observers had long experience with 5-min counts. Less experienced observers did some initial joint counts on site for standardization, and in 2019–2020, uncertain calls were recorded for later confirmation. The first study (1978–1982) used 15 observers with two to four observers on any one date, and several observers contributed counts on many dates over 2 or 3 yr. The second study used four observers in total with two on any date, and one of the observers counted across all 6 yr. The third study used seven observers, with LR involved in nearly all count sessions. Observer was further standardized in the analysis (see below).
Mountain Beech Seedfall and Pest Control Programs
Annual seedfall of mountain beech has been measured at Broken River since 1965 (Allen and Platt 1990). Seedfall was collected in eight seed trays spaced 40 m apart along a transect at 1,050 m elevation (Fig. 1). Each tray was funnel shaped with a catch area of 0.28 m². Seedfall values are annual seeds per m² averaged across the eight seed trays (Table 2). Seedfall at 1,050 m is highly correlated with seedfall at other altitudes in the same catchment (Allen and Platt 1990).
Different pest management was carried out over the last 40 yr, at first intermittently, then since 2007, in a sustained way. Before 2007, pest control was performed as part of research projects (King 1983, Spurr 2000, Kelly et al. 2005) that targeted stoats in the Broken River catchment (Table 2). Stoats were trapped from November 1973 to May 1978, poisoned in 1994, and trapped in spring 2000–2001. Brushtail possums are present in Craigieburn Forest Park, but only limited possum trapping has been carried out since the 1980s (Kelly et al. 2005; Ray Goldring, personal communication) due to the risk possum traps pose to Kea. Since 2007, intensive and sustained pest mammal trapping programs have been run by community groups, the Canterbury Environmental Trust and New Zealand Conservation Trust, using DOC200 kill traps, which target stoats, but also catch other mammals including weasels, rats, hedgehogs, and a few ferrets, feral cats, and possums (Harris et al. 2022). No rats at all were caught in the 1980s (King 1983), and only a single ship rat was recorded in 1999–2004 (Kelly et al. 2005). However, three ship rats were caught in 2008–2009, and since then, 383 more have been caught in 2010–2019 (Harris et al. 2022). A Vespula wasp management program has been carried out since 2015 using poison bait (Vespex) (Crossland 2017).
Statistical Analysis
We analyzed trends over time for 15 bird species: the nine most commonly recorded native species and the six most common exotics. For each bird species, the response variable was the number of birds per 5-min count. Fixed effects were either study, year, or seedfall, along with season, elevation, and site. Study was used as a fixed effect with three levels (with the 1999–2004 study as the reference period) to explore the longer-term bird species trends, whereas year was used in separate models to examine bird population trends across individual years. Seedfall was used to investigate the relationship between mountain beech masting and bird counts.
We used generalized linear mixed models (GLMMs) to examine factors likely to influence 5-min bird counts (Dawson and Bull 1975, Dawson et al. 1978, Hartley 2012) and to allow for the unbalanced study design using fixed and random terms. For location, we used a fixed term for site (Broken River, Cheeseman, or Craigieburn) and present fitted values for Broken River, which had 88% of the counts. Within sites, marked bird count stations were established independently in each study period, so we fitted random terms at two nested spatial scales: subsite (the particular road or track; Fig. 1) and individual stations (constant within a period). The final model used either station nested within subsite, or only station, whichever gave a lower Akaike Information Criterion (AIC) (Sakamoto et al. 1986), as listed in Append 1: Table A1. We also included a random term for observer. Observers often counted across multiple dates within a study, but no observers spanned all three studies. Thus, the random term corrected for observer differences among dates within a study, whereas comparisons between studies used the observer-corrected fitted means from each of the studies.
Elevation and season were included as fixed effects (Elliott et al. 2010). Although the four seasons were not surveyed evenly, the large number of counts performed every 2 mo in 1980–1982 allowed us to measure seasonal trends in each bird species and use this to correct for any differences in seasonal spread of counts among different time periods. Winter (June–August in the southern hemisphere) counts were common in 1978–1982 (n = 2800), but not performed in 1999–2004 and rare (n = 40) in 2019–2020, so we added June (early winter) bird counts into the “autumn” category and August (late winter) counts into “spring” (Table 1). Although spring counts were included in the analysis (see Append. 1: Tables A2, A3 for detailed spring outputs), we present bird fitted values only for autumn and summer as these seasons had counts performed in all three studies (Table 2). For year-to-year comparisons, we grouped counts by “seed year” corresponding to 12 mo from March to the following February. This was to ensure that counts from one summer (December–February) were included in a single year, and to allow for possible impacts of variable mountain beech seed crops among years. The seed year started in March when mountain beech seed begins to fall, with a direct or delayed impact on some bird species, rodent, and stoat numbers (King 1983, O'Donnell and Phillipson 1996, O'Donnell and Hoare 2012).
Generalized linear mixed models were run using the glmmTMB package (Brooks et al. 2017) in R software version 3.5.1 (R Core Development Team 2018), initially with a Poisson error distribution. For some bird species, there was significant overdispersion with a large number of zeros, so we also explored negative binomial, zero-inflated Poisson or zero-inflated negative binomial error distributions, and selected the best model using AIC (see detailed formula in Append. 1: Table A1). In four species, a zero-inflated model (ZIP or ZINB) gave the best AIC for the study period analysis, but could not be fitted to the seed year analysis, which therefore had to use a Poisson GLMM (Append. 1: Table A1). This means the fitted values of the study and seed year models were not directly comparable, but this only caused noticeable differences for Rifleman, as discussed below. Each model was checked for overdispersion and zero inflation using the DHARMa package in R (Hartig 2020).
RESULTS
We detected 32 bird species (19 natives and 13 exotics), with Bellbird the most often recorded overall, followed by Silvereye (Zosterops lateralis) and two exotic species, Redpoll (Acanthis flammea) and Chaffinch (Fringilla coelebs) (Table 3). Although no Kea were recorded in 2019–2020 counts, residents at Castle Hill village and personal observation confirmed that some Kea remained around Craigieburn Forest Park in summer 2019–2020. The analysis of fitted values by seed year corrects for unbalanced sampling across seasons, sites (Broken River, Cheeseman, and Craigieburn), and observers, which affect the raw means in Table 3, so the fitted values allow better comparison across years. The analyses used either seed year (Figs. 2–4) or study period (Table 5). Species whose population abundance changed little across the three study periods, such as Silvereye, Blackbird (Turdus merula), or Long-tailed Cuckoo still had significant variation among years (Fig. 2–4; Append. 1: Tables A2, A3). Interestingly, all common exotic passerines (Blackbird, Chaffinch, Redpoll, Dunnock (Prunella modularis), Greenfinch (Carduelis chloris), Goldfinch (Carduelis carduelis)) showed significant increases in relation to beech seed crops, but this was not apparent in the native species, where only Bellbird showed a significant relationship with seedfall (Table 4).
The key test for long-term trends is the study period analysis, which compares between longer time blocks. Bellbird was the only species showing a significant increase over the three studies (Table 5; Append. 1: Table A2). No species declined throughout the three study periods, but counts of Brown Creeper and Grey Warbler (Gerygone igata) were significantly lower at the end than at the start. Brown Creeper counts were stable from 1978–1982 to 1999–2004 but then declined significantly by 2019–2020. Grey Warbler counts decreased between 1978–1982 and 1999–2004 but then did not change from 1999–2004 to 2019–2020. Four species (Rifleman, Tomtit, Chaffinch, and Dunnock) decreased between 1978–1982 and 1999–2004, then recovered by 2019–2020. The significant increase of Rifleman from 1999–2004 to 2019–2020 (Table 5) seems at odds with the low fitted values for 2019–2020 in Fig. 2, but the latter come from a Poisson GLMM, whereas the analysis by study period used a zero-inflated Poisson. Because of the different way the ZIP model handles zeros, the study period estimated fitted values for Rifleman were higher in 2019–2020 (summer 0.69 per count and autumn 0.20) than in 1999–2004 (summer 0.41, autumn 0.19). Significantly more Kea were observed in 1999–2004 than in 1978–1982. The complete absence of Kea during the 2019–2020 study prevented us from obtaining any significant results between 2019–2020 and the two other study periods. Six species (Silvereye, Fantail (Rhipidura fuliginosa), Long-tailed Cuckoo, Redpoll, Blackbird, and Greenfinch) showed no significant changes between study periods (Table 5). Overall, between 1978–1982 and 1999–2004 more species decreased than increased (five vs. two species), whereas from 1999–2004 to 2019–2020 there were more increases (six) than decreases (one).
DISCUSSION
Study Limitations and Long-term Study Designs
The maintenance of regular long-term monitoring of birds and seedfall combined with pest control data is important for improving pest management, such as the Predator-Free New Zealand 2050 campaign (Owens 2017). Our study highlights the challenges faced when measuring biological trends over long periods. Three issues limit the strength of inferences we can make: the time of year of sampling varied, the exact locations of bird count stations were not available for earlier studies, and no observers counted in more than one of the three time periods. We discuss the possible impact of these issues and make recommendations to reduce their effects in future work.
Sampling at different times of year affects bird counts because conspicuousness (detectability) varies seasonally for some species (Dawson et al. 1978). Sampling in the same months each year would limit the influence of this confounding factor. In most planned long-term studies in New Zealand, counts have been made annually in October–December (austral spring–early summer) (Hoare et al. 2012, O'Donnell and Hoare 2012). However, in opportunistic long-term studies such as ours, which build on counts made in earlier studies, count dates are necessarily dictated by the dates used in the earlier studies (Pierce et al. 1993, Smith and Westbrooke 2004, Spurr and Anderson 2004, Elliott et al. 2010, Barnett 2011). In our study, the three studies sampled in different combinations of months (see Methods). We could have compared counts made only in the same months, but this would have drastically reduced the number of counts available for analysis. Instead, we chose to use all counts and include seasonal variation in our modeling. This was possible because the first time period (1978–1982) sampled intensively through all seasons over several years. Thus, we were able to correct for season when comparing changes across years.
The exact location of bird count stations can be important because some areas (subsites) have different bird abundances, due to variations in altitude, vegetation type, and other factors. Statistically, this can be allowed for at various scales by including random terms for subsite, track and/or count station, which increases the power of the analysis to detect changes over time. In our case, count stations were constant within a study, but different between studies, because the exact count station locations for the first two studies were not recorded. We were able to standardize for spatial variation at the subsite level because the three studies sampled along many of the same tracks. This controlled for altitude and vegetation effects, but not for any local effects at individual count stations between time periods. A key lesson for future studies would be to archive the exact count station locations.
More difficult is allowing for different observers over time. Variation in bird counts among observers has long been of concern (Faanes and Bystrak 1981, Lindenmayer et al. 2009). This problem is inevitable in very long studies, where the available observers will necessarily change over time (e.g., Elliott et al. 2010, Graham et al. 2013, Miskelly 2018, Ralph et al. 2020). Even if the same observers were present throughout, their counts likely alter over time as they become more experienced and their high-frequency hearing is weakened (Faanes and Bystrak 1981). The most effective ways to decrease this variation are to use experienced observers, to have training on-site initially for standardization, and to have each site counted by several different observers on different days (Faanes and Bystrak 1981, Cunningham et al. 1999, Lindenmayer et al. 2009). All those methods were used in our study. The resulting average counts have been found to be similar enough that adjusting for observer differences would have relatively little effect (Lindenmayer et al. 2009).
Using consistent sampling design, and carefully archiving raw data with open access for interested researchers would facilitate future long-term studies, as recommended by Hartley (2012). Standardized methods are already used by some large-scale programs such as the National Vegetation Survey databank in New Zealand (Wiser et al. 2001) and the Forest Inventory and Analysis program in the USA (Bechtold and Patterson 2005). Similar approaches should be extended to other taxa at national scales (Bellingham et al. 2020).
Long-term Changes at Craigieburn Forest Park
The long-term trends in bird counts 1978–2020 were different for native and exotic birds. Only one native species (Bellbird) showed a consistent increase in counts from the start to the end of our study, and two (Brown Creeper and Grey Warbler) showed a decrease. Most native species fluctuated, with more increasing later in the study, coincident with regular pest control. In contrast, the exotic birds seemed to largely fluctuate in response to beech mast seed years. Although confounding variables such as seasons or observers were considered in our analysis, the results need to be interpreted carefully due to the complexity of the data set. As this study was observational rather than manipulative, the causes of trends cannot be established with certainty, but some links with the literature can be made.
The increase in Bellbird counts across the three study periods is likely in part due to stoat control programs, because the benefits of pest control for Bellbird populations are well documented (Graham and Veitch 2002, Kelly et al. 2005, O'Donnell and Hoare 2012, Graham et al. 2013, Miskelly 2018). The small-scale stoat control carried out by Kelly et al. (2005) during two breeding seasons (summer 2000–2001 and 2001–2002) at Craigieburn Forest Park revealed that stoat trapping can increase Bellbird nesting success and 5-min bird counts. Bellbird is a relatively long-lived species (5–10 yr), with adults less vulnerable to mammal predation than eggs and chicks (Kelly et al. 2005). Under intermittent mammal pest control, adult Bellbirds are likely to survive until the next pest control event (Kelly et al. 2005, Parlato et al. 2015, Walker et al. 2019). In the absence of mammal pest control in Nelson Lakes National Park, Bellbirds declined (Elliott et al. 2010).
Two native species declined long term, Brown Creeper and Grey Warbler, but only the first causes much concern. Brown Creeper is present across much of the South Island’s native forest. Although Robertson et al. (2007) reported a decrease in range between the two Bird Atlases, the analysis by Walker and Monks (2018) showed a stable range between 1969–1979 and 1999–2004, and other work showed Brown Creepers benefit from mammal pest control (O'Donnell and Hoare 2012). The decline at Craigieburn Forest Park could be related to one-off events rather than a slow declining process. Although we sampled from March 2019 to January 2020, this was all still in a single seed year, and further counts would be required to confirm whether Brown Creepers stay at lower densities. Declines in Brown Creeper populations could negatively affect Long-tailed Cuckoos because the cuckoo is a brood parasite of just two species as hosts: Brown Creeper and its now-rare congener the Mohua (Robertson et al. 2001).
The decline in Grey Warblers is less concerning, and may be related to competition with bird species that increase after mammal pest control, such as Bellbirds. Competitive relationships for resources have been previously observed between Grey Warbler (and also Fantail and Silvereye) and various other native species, including Bellbird (Innes et al. 2010, Miskelly 2018). Although Bellbirds increased and Grey Warblers decreased, we did not observe declines in the counts of Fantails or Silvereyes, so the causes of Grey Warbler decline remain uncertain. The decline is of low concern as Grey Warbler (along with Fantail and Silvereye) are common in natural and modified habitats throughout New Zealand and appear to be less vulnerable to predation by mammals than most endemic birds (Ruffell and Didham 2017, Miskelly 2018).
The results for Rifleman are complex. The analysis by study period showed that Rifleman increased significantly between 1999–2004 and 2019–2020, whereas the seed year analysis showed low fitted means for Rifleman in the 2019 seed year. The raw data showed low counts in autumn 2019, but relatively high counts in January 2020. The difference between analysis by seed year vs. study period can be explained by inherent differences between a zero-inflated Poisson model (used in the study period analysis) and a plain Poisson model (used in the seed year analysis where a zero-inflated model did not converge). The zero-inflated model should be more appropriate as Rifleman were often seen in family groups, and for study period, the zero-inflated model had the best AIC. The plain Poisson is expected to predict lower fitted means (Brooks et al. 2017). We consider that the Rifleman population did recover by 2020 from its significant decline between 1978–1982 and 1999–2004, probably benefiting from the predator control at Craigieburn Forest Park. Results of Rifleman population responses to mammal pest control in other studies are variable, with either a positive response (O'Donnell and Hoare 2012, Elliott and Kemp 2016) or a decline after pest control (Vianen et al. 2018). Rifleman is a cavity-nesting species, which makes it vulnerable to stoat and rat predation (O'Donnell 1996, Parlato et al. 2015, Walker et al. 2019). Tomtit showed a similar general trend to Rifleman, also declining at Craigieburn from 1978–1982 to 1999–2004, and increasing from 1999–2004 to 2019–2020, perhaps also benefiting from predator control.
The exotic bird populations in this study appeared to be stable over long time scales, but showed pronounced short-term increases during mountain beech high-seed years. All six exotic species can be common in native forest, but they (especially the four finches) are also often abundant in modified habitats including grasslands (Case 1996, Barnagaud et al. 2014). Because Craigieburn Forest Park adjoins exotic grasslands, increased counts in high-seed years may be due to birds moving from grasslands into the forest to feed on beech seed and/or associated increases in arthropods (Alley et al. 2001, O'Donnell and Hoare 2012). In contrast to the exotic birds, only one native species (Bellbird) increased in high-seed years. The Bellbird relationship with seedfall may be coincidental, as Bellbirds do not feed directly on beech seed. Of native birds at Craigieburn, only Parakeets (Cyanoramphus spp.) eat beech seed, and they were too uncommon to analyze. The responses of native birds to masting are complex and dominated by lagged effects of mammalian pest irruptions (O'Donnell and Phillipson 1996, Innes et al. 2010, Elliott and Kemp 2016, Vianen et al. 2018). However, we could not test this as intermittent mammal pest control meant that seedfall would not be a good predictor of mammal pest densities, and we had no direct measurements of mammal abundance.
Considered broadly, the early part of our study had intermittent mammal pest control (stoat control in 9 yr between 1973 and 2004). This is more than most conservation lands in New Zealand, which get no mammal pest control at all (Wright 2011). Mount Misery, the location of the study by Elliott et al. (2010), is an example. But even with modest pest control in Craigieburn Forest Park, three of nine native species declined during the first 25 yr. By contrast, our final counts in 2019–2020 came after 13 yr of sustained stoat trapping, and three native bird species increased (Bellbird, Rifleman, Tomtit), with only one decreasing (Brown Creeper). Without manipulations, replication or non-treatment areas, we cannot prove mammal pest control caused the increases, but our data are generally consistent with other studies (Binny et al. 2020) showing benefits of mammal pest control in New Zealand forests for bird species undergoing gradual decline, especially those in endemic genera like Rifleman. Exotic birds showed a different pattern, as previous studies have also found (Binny et al. 2020), with increases at the end of our study most likely caused by the large mountain beech seed crop in 2019.
One point of concern is the recent arrival of the ship rat at Craigieburn around 2010, as this is one of the worst bird predators in New Zealand (Innes et al. 2010). Ship rats were completely absent during the first study period and almost completely absent during the second (one individual rat caught in 2000–2001). Since 2010, pest control programs at Craigieburn are regularly catching ship rats, showing that they are now well established (Harris et al. 2022). Ship rat establishment may have been favored by climate change (Allen et al. 2014, Walker et al. 2019, Harris et al. 2022) and/or by low stoat density. Whitau et al. (in press) found that stoat trapping in South Island beech forests increased rat density, although Ruscoe et al. (2011) found no evidence for the latter in North Island experiments. Although Rifleman and Tomtit populations showed an increase by 2019 in the presence of the ship rat invasion, we could expect a stronger recovery as well as an increase of Brown Creeper if pest control programs were implemented to target ship rats (Graham and Veitch 2002, Miskelly and Robertson 2002).
Birds provide important ecosystem services (Şekercioğlu et al. 2016) including seed dispersal and pollination (Kelly et al. 2010, Anderson et al. 2011). Many endemic New Zealand plants have a close mutualistic relationship with native birds, with about 30% of tree species having bird-visited flowers and 59% having fleshy fruits (Kelly et al. 2010). Bellbird, Silvereye, and Tui are common and widespread species that provide both pollination and seed dispersal services (Anderson et al. 2006, Kelly et al. 2010). In New Zealand, population declines among native birds due to predation and competition affect these ecosystem services (Anderson et al. 2011). In some cases, pollination or dispersal services improved following mammal pest control (Iles and Kelly 2014, Bombaci et al. 2021). However, Kelly et al. (2005) found no evidence of a short-term increase in pollination service for mistletoe (Peraxilla tetrapetala) in Craigieburn after stoat control, despite a 79% increase in Bellbird abundance. They suggested that Bellbird density may have been too low to generate a detectable change in pollination rates. At Craigieburn, Bellbird counts have increased over the last 40 yr, including a significant increase post-2004, and by summer 2019–2020, there were around 3.4 Bellbirds per 5-min bird count compared with 1.5 in 1978–1982 (Append. 1: Table A4). Although we have not measured current pollination service, Craigieburn now has relatively high densities of the two key local pollinators and frugivores (Kelly et al. 2006), with a growing Bellbird population and stable Silvereye population.
In conclusion, long-term bird population changes in an area of native forest with some mammal pest management varied between native and exotic species at Craigieburn Forest Park. These results suggest that stoat control programs may have benefited native birds, especially in more recent years when trapping has been more intensive and continuous. However, the recent establishment of ship rats at the site could put new predation pressure on local birds unless ship rat management programs are initiated. This study clearly demonstrates that still-widespread endemic species may need protection because their populations could lack resilience against unpredictable events such as the arrival of pathogens or predators.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.AUTHOR CONTRIBUTIONS
LR, DK, DJF, and EGB conceptualized the manuscript. EBS, LR, DK, DJF, and RBA contributed to data curation and investigation. LR performed the formal analysis with contributions from DK and EGB, who also contributed to their interpretation; LR wrote the manuscript with contributions from all authors DK, EBS, DJF, RBA, and EGB. All authors gave their approval for publication.
ACKNOWLEDGMENTS
We thank all those who helped with bird counts, including Malcolm Anderson, Mike Arbuckle, Steve Anderson, Kev Drew, John Eggleston, Howard Fitzgerald, Steve Hough, Sue Kingsford, Dave Lewitt, Jill Murray, Alan Rose, Lou Sanson, Bill Simes, Steve Sutton, and Dave Thomas in 1978–1982, Brian Karl, Peter Wilson, Jo Reese, and Bruce Thomas in 1999–2004, and Archie MacFarlane, Diane Cowan, Katrina McCallum, and Robyn White in 2019–2020. We thank Ray Goldring and the Canterbury Environmental Trust for trapping data and field accommodation, and Elena Moltchanova for advice on statistics. This Craigieburn bird count project was supported by Scion (New Zealand Forest Research Institute) with funding from the MBIE program "BEST: Building biodiversity into an ecosystem service-based approach for resource management" (MBIE contract C09X1307) and a grant to DF from the Organization for Economic Cooperation and Development (OECD) in 2019–2020.
LITERATURE CITED
Allen, R. B., J. M. Hurst, J. Portier, and S. J. Richardson. 2014. Elevation‐dependent responses of tree mast seeding to climate change over 45 years. Ecology and Evolution 4(18):3525-3537. https://doi.org/10.1002/ece3.1210
Allen, R. B., and K. H. Platt. 1990. Annual seedfall variation in Nothofagus solandri (Fagaceae), Canterbury, New Zealand. Oikos 57:199-206. https://doi.org/10.2307/3565940
Alley, J. C., P. H. Berben, J. S. Dugdale, B. M. Fitzgerald, P. I. Knightbridge, M. J. Meads, and R. A. Webster. 2001. Responses of litter‐dwelling arthropods and house mice to beech seeding in the Orongorongo Valley, New Zealand. Journal of the Royal Society of New Zealand 31(2):425-452. https://doi.org/10.1080/03014223.2001.9517663
Anderson, S. H., D. Kelly, J. J. Ladley, S. Molloy, and J. Terry. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science 331(6020):1068-1071. https://doi.org/10.1126/science.1199092
Anderson, S. H., D. Kelly, A. W. Robertson, J. J. Ladley, and J. G. Innes. 2006. S04-3 birds as pollinators and dispersers: a case study from New Zealand. Acta Zoologica Sinica 52(Suppl.1):112-115.
Barnagaud, J.-Y., L. Barbaro, J. Papaix, M. Deconchat, and E. G. Brockerhoff. 2014. Habitat filtering by landscape and local forest composition in native and exotic New Zealand birds. Ecology 95(1):78-87. https://doi.org/10.1890/13-0791.1
Barnett, C. 2011. Changes in the observed bird abundance in a modified forest at Kowhai Bush, Kaikoura. Notornis 58(3-4):131-138.
Bechtold, W. A., and P. L. Patterson. 2005. The enhanced forest inventory and analysis program—national sampling design and estimation procedures. U.S. Department of Agriculture, Forest Service, Southern Research Station, Asheville, North Carolina, USA. https://doi.org/10.2737/SRS-GTR-80
Beggs, J. 2001. The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biological Conservation 99(1):17-28. https://doi.org/10.1016/S0006-3207(00)00185-3
Bellingham, P. J., S. J. Richardson, A. M. Gormley, R. B. Allen, A. Cook, P. N. Crisp, D. M. Forsyth, M. S. McGlone, M. McKay, C. J. MacLeod, P. van Dam-Bates, and E. F. Wright. 2020. Implementing integrated measurements of essential biodiversity variables at a national scale. Ecological Solutions and Evidence 1(2): e12025. https://doi.org/10.1002/2688-8319.12025
Binny, R. N., J. Innes, N. Fitzgerald, R. Pech, A. James, R. Price, C. Gillies, and A. E. Byrom. 2020. Long-term biodiversity trajectories for pest-managed ecological restorations: eradication vs. suppression. Ecological Monographs 91(2): e01439. https://doi.org/10.1002/ecm.1439
Bombaci, S. P., J. Innes, D. Kelly, V. Flaherty, and L. Pejchar. 2021. Excluding mammalian predators increases bird densities and seed dispersal in fenced ecosanctuaries. Ecology 102(6): e03340. https://doi.org/10.1002/ecy.3340
Bowler, D. E., H. Heldbjerg, A. D. Fox, M. de Jong, and K. Böhning‐Gaese. 2019. Long-term declines of European insectivorous bird populations and potential causes. Conservation Biology 33(5):1120-1130. https://doi.org/10.1111/cobi.13307
Brooks, M. E., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Machler, and B. M. Bolker. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal 9(2):378-400. https://doi.org/10.3929/ethz-b-000240890
Case, T. J. 1996. Global patterns in the establishment and distribution of exotic birds. Biological Conservation 78(1-2):69-96. https://doi.org/10.1016/0006-3207(96)00019-5
Crossland, A. 2017. Craigieburn wasp control project - bird monitoring report No. 2, spring 2016. Waimakariri Environment and Recreation Trust, Christchurch, New Zealand.
Cunningham, R. B., D. B. Lindenmayer, H. A. Nix, and B. D. Lindenmayer. 1999. Quantifying observer heterogeneity in bird counts. Australian Journal of Ecology 24(3):270-277. https://doi.org/10.1046/j.1442-9993.1999.00971.x
Dawson, D. G., and P. C. Bull. 1975. Counting birds in New Zealand forests. Notornis 22(2):101-109.
Dawson, D. G., P. J. Dilks, P. D. Gaze, J. G. R. McBurney, and P. R. Wilson. 1978. Seasonal differences in bird counts in forests near Reefton, South Island, New Zealand. Notornis 25(4):257-278.
Elliott, G., and J. Kemp. 2016. Large-scale pest control in New Zealand beech forests. Ecological Management and Restoration 17(3):200-209. https://doi.org/10.1111/emr.12227
Elliott, G. P., P. J. Dilks, and C. F. J. O'Donnell. 1996. The ecology of Yellow‐crowned Parakeets (Cyanoramphus auriceps) in Nothofagus forest in Fiordland, New Zealand. New Zealand Journal of Zoology 23(3):249-265. https://doi.org/10.1080/03014223.1996.9518084
Elliott, G. P., P. R. Wilson, R. H. Taylor, and J. R. Beggs. 2010. Declines in common, widespread native birds in a mature temperate forest. Biological Conservation 143(9):2119-2126. https://doi.org/10.1016/j.biocon.2010.05.022
Faanes, C. A., and D. Bystrak. 1981. The role of observer bias in the North American Breeding Bird Survey. Studies in Avian Biology 6:353-359. URL: https://sora.unm.edu/sites/default/files/SAB_006_1981_P353-359%20Part%207%20The%20Role%20of%20Observer%20Bias%20in%20the%20North%20American...%20Craig%20A.%20Faanes%20Danny%20Bystrak.pdf
Fea, N., W. Linklater, and S. Hartley. 2020. Responses of New Zealand forest birds to management of introduced mammals. Conservation Biology 35(1):35-49. https://doi.org/10.1111/cobi.13456
Graham, M., D. Veitch, G. Aguilar, and M. Galbraith. 2013. Monitoring terrestrial bird populations on Tiritiri Matangi Island, Hauraki Gulf, New Zealand, 1987-2010. New Zealand Journal of Ecology 37(3):359-369. URL: http://www.jstor.org/stable/44945234
Graham, M. F., and C. R. Veitch. 2002. Changes in bird numbers on Tiritiri Matangi Island, New Zealand, over the period of rat eradication. Pages 120-123 in C. R. Veitch and M. N. Cloud, editors. Turning the tide: the eradication of invasive species. IUCN SSC Invasive Species Specialist Group, Gland, Switzerland and Cambridge, UK.
Harris, H. A. L., D. Kelly, J. Innes, and R. B. Allen. 2022. Invasive species and thermal squeeze: distribution of two invasive predators and drivers of ship rat (Rattus rattus) invasion in mid-elevation Fuscospora forest. Biological Invasions:1-13. https://doi.org/10.1007/s10530-022-02789-4
Hartig, F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed). Regression models. R package version 0.3.2.0. URL: https://CRAN.R-project.org/package=DHARMa
Hartley, L. J. 2012. Five-minute bird counts in New Zealand. New Zealand Journal of Ecology 36(3):1. URL: https://newzealandecology.org/nzje/3041.pdf
Hoare, J. M., A. Monks, and C. F. J. O'Donnell. 2012. Can correlated population trends among forest bird species be predicted by similarity in traits? Wildlife Research 39(6):469-477. https://doi.org/10.1071/WR11087
Holdaway, R. N. 1989. New Zealand's pre-human avifauna and its vulnerability. New Zealand Journal of Ecology 12(s):11-25. URL: https://newzealandecology.org/nzje/1856.pdf
Holdaway, R. N. 1999. Introduced predators and avifaunal extinction in New Zealand. Pages 189-238 in R. D. E. MacPhee, editor. Extinctions in near time. Springer, Boston, Massachusetts, USA. https://doi.org/10.1007/978-1-4757-5202-1_9
Iles, J. M., and D. Kelly. 2014. Restoring bird pollination of Fuchsia excorticata by mammalian predator control. New Zealand Journal of Ecology 38(2):297-306. URL: https://newzealandecology.org/nzje/3131.pdf
Innes, J., D. Kelly, J. M. C. Overton, and C. Gillies. 2010. Predation and other factors currently limiting New Zealand forest birds. New Zealand Journal of Ecology 34(1):86-114. URL: https://newzealandecology.org/nzje/2911.pdf
Kelly, D., C. Brindle, J. J. Ladley, A. W. Robertson, F. W. Maddigan, J. Butler, T. Ward-Smith, D. J. Murphy, and L. A. Sessions. 2005. Can stoat (Mustela erminea) trapping increase bellbird (Anthornis melanura) populations and benefit mistletoe (Peraxilla tetrapetala) pollination? New Zealand Journal of Ecology 29(1):69-82. URL: https://newzealandecology.org/nzje/2249.pdf
Kelly, D., A. Geldenhuis, A. James, E. Penelope Holland, M. J. Plank, R. E. Brockie, P. E. Cowan, G. A. Harper, W. G. Lee and M. J. Maitland. 2013. Of mast and mean: differential‐temperature cue makes mast seeding insensitive to climate change. Ecology Letters 16(1):90-98. https://doi.org/10.1111/ele.12020
Kelly, D., W. D. Koenig, and A. M. Liebhold. 2008. An intercontinental comparison of the dynamic behavior of mast seeding communities. Population Ecology 50:329-342. https://doi.org/10.1007/s10144-008-0114-4
Kelly, D., J. J. Ladley, A. W. Robertson, S. H. Anderson, D. M. Wotton, and S. K. Wiser. 2010. Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit-dispersal in New Zealand. New Zealand Journal of Ecology 34(1):66-85. URL: https://newzealandecology.org/nzje/2918.pdf
Kelly, D., A. W. Robertson, J. J. Ladley, S. H. Anderson, and R. J. McKenzie. 2006. Relative (un) importance of introduced animals as pollinators and dispersers of native plants. Pages 227-245 in R. B. Allen and W. G. Lee, editors. Biological invasions in New Zealand. Springer, Berlin, Heidelberg, Germany. https://doi.org/10.1007/3-540-30023-6_15
Kelly, D., and J. J. Sullivan. 2010. Life histories, dispersal, invasions, and global change: progress and prospects in New Zealand ecology, 1989-2029. New Zealand Journal of Ecology 34(1):207-217. URL: https://newzealandecology.org/nzje/2919.pdf
King, C. M. 1983. The relationships between beech (Nothofagus sp.) seedfall and populations of mice (Mus musculus), and the demographic and dietary responses of stoats (Mustela erminea), in three New Zealand forests. The Journal of Animal Ecology 52(1):141-166. https://doi.org/10.2307/4593
Lester, P. J., J. R. Beggs, R. L. Brown, E. D. Edwards, R. Groenteman, R. J. Toft, A. Twidle, and D. Ward. 2013. The outlook for control of New Zealand’s most abundant, widespread and damaging invertebrate pests: social wasps. New Zealand Science Review 70(4):54-62. URL: http://hdl.handle.net/2292/35313
Lindenmayer, D. B., J. T. Wood, and C. MacGregor. 2009. Do observer differences in bird detection affect inferences from large-scale ecological studies? Emu - Austral Ornithology 109(2):100-106. https://doi.org/10.1071/MU08029
Miskelly, C. M. 2018. Changes in the forest bird community of an urban sanctuary in response to pest mammal eradications and endemic bird reintroductions. Notornis 65(3):132-151.
Miskelly, C., and H. Robertson. 2002. Response of forest birds to rat eradication on Kapiti Island, New Zealand. Department of Conservation, Wellington, New Zealand.
Moorhouse, R., T. Greene, P. Dilks, R. Powlesland, L. Moran, G. Taylor, A. Jones, J. Knegtmans, D. Wills, and M. Pryde. 2003. Control of introduced mammalian predators improves kaka Nestor meridionalis breeding success: reversing the decline of a threatened New Zealand parrot. Biological Conservation 110(1):33-44. https://doi.org/10.1016/S0006-3207(02)00173-8
Murphy, D. J., and D. Kelly. 2003. Seasonal variation in the honeydew, invertebrate, fruit and nectar resource for bellbirds in a New Zealand mountain beech forest. New Zealand Journal of Ecology 27(1):11-23. URL: https://newzealandecology.org/nzje/2174.pdf
O’Donnell, C. F. J. 1996. Predators and the decline of New Zealand forest birds: an introduction to the hole‐nesting bird and predator programme. New Zealand Journal of Zoology 23(3):213-219. https://doi.org/10.1080/03014223.1996.9518080
O’Donnell, C. F. J., and J. M. Hoare. 2012. Quantifying the benefits of long-term integrated pest control for forest bird populations in a New Zealand temperate rainforest. New Zealand Journal of Ecology 36(2):131-140. URL: https://newzealandecology.org/nzje/3022.pdf
O’Donnell, C. F. J., and S. M. Phillipson. 1996. Predicting the incidence of mohua predation from the seedfall, mouse, and predator fluctuations in beech forests. New Zealand Journal of Zoology 23(3):287-293. https://doi.org/10.1080/03014223.1996.9518087
Owens, B. 2017. Behind New Zealand’s wild plan to purge all pests. Nature 541:148-150. https://doi.org/10.1038/541148a
Parlato, E. H., D. P. Armstrong, and J. G. Innes. 2015. Traits influencing range contraction in New Zealand’s endemic forest birds. Oecologia 179(2):319-328. https://doi.org/10.1007/s00442-015-3330-6
Pierce, R. J., R. Atkinson, and E. Smith. 1993. Changes in bird numbers in six Northland forests 1979-1993. Notornis 40:285-293.
R Core Development Team. 2018. R: a language and environment for statistical computing. R Project for Statistical Computing, Vienna, Austria.
Ralph, C. J., C. P. Ralph, and L. L. Long. 2020. Towards the reestablishment of community equilibrium of native and non-native landbird species in reponse to pest control on islands in the Eastern Bay of Islands, New Zealand. Notornis 67:437-450. URL: https://www.fs.usda.gov/treesearch/pubs/60694
Robertson, C. J. R., P. Hyvönen, M. J. Fraser, and C. R. Pickard. 2007. Atlas of bird distribution in New Zealand 1999-2004. The Ornithological Society of New Zealand, Wellington, New Zealand.
Robertson, H. A., K. Baird, J. E. Dowding, G. Elliott, R. A. Hitchmough, C. M. Miskelly, N. McArthur, C. F. J. O'Donnell, P. M. Sagar, and R. P. Scofield. 2017. Conservation status of New Zealand birds, 2016. New Zealand threat classification series 19. Department of Conservation, Wellington, New Zealand. URL: https://www.doc.govt.nz/globalassets/documents/science-and-technical/nztcs19entire.pdf
Robertson, H. A., B. D. Heather, and D. J. Onley. 2001. The hand guide to the birds of New Zealand. Oxford University Press, Oxford, UK.
Rosenberg, K. V., A. M. Dokter, P. J. Blancher, J. R. Sauer, A. C. Smith, P. A. Smith, J. C. Stanton, A. Panjabi, L. Helft, and M. Parr. 2019. Decline of the North American avifauna. Science 366(6461):120-124. https://doi.org/10.1126/science.aaw1313
Ruffell, J., and R. K. Didham. 2017. Conserving biodiversity in New Zealand’s lowland landscapes: does forest cover or pest control have a greater effect on native birds? New Zealand Journal of Ecology 41(1):23-33. https://doi.org/10.20417/nzjecol.41.12
Ruscoe, W. A., D. S. L. Ramsey, R. P. Pech, P. J. Sweetapple, I. Yockney, M. C. Barron, M. Perry, G. Nugent, R. Carran, R. Warne, C. Brausch, and R. P. Duncan. 2011. Unexpected consequences of control: competitive vs. predator release in a four‐species assemblage of invasive mammals. Ecology Letters 14(10):1035-1042. https://doi.org/10.1111/j.1461-0248.2011.01673.x
Russell, J. C., J. G. Innes, P. H. Brown, and A. E. Byrom. 2015. Predator-free New Zealand: conservation country. BioScience 65(5):520-525. https://doi.org/10.1093/biosci/biv012
Sakamoto, Y., M. Ishiguro, and G. Kitagawa. 1986. Akaike information criterion statistics. D. Reidel Publishing Company, Dordrecht, The Netherlands.
Schauber, E. M., D. Kelly, P. Turchin, C. Simon, W. G. Lee, R. B. Allen, I. J. Payton, P. R. Wilson, P. E. Cowan, and R. E. Brockie. 2002. Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83(5):1214-1225. https://doi.org/10.2307/3071937
Şekercioğlu, Ç. H., G. C. Daily, and P. R. Ehrlich. 2004. Ecosystem consequences of bird declines. Proceedings of the National Academy of Sciences 101(52):18042-18047. https://doi.org/10.1073/pnas.0408049101
Şekercioğlu, Ç. H., D. G. Wenny, and C. J. Whelan. 2016. Why birds matter: avian ecological function and ecosystem services. University of Chicago Press, Chicago, Illinois, USA. https://doi.org/10.7208/chicago/9780226382777.001.0001
Shanks, A., D. Glenny, R. Gibson, K. Rosser, D. Roozen, S. Phillipson, J. Steven, and J. Arand. 1990. Coleridge, Craigieburn and Cass ecological districts. Department of Conservation, Wellington, New Zealand.
Smaill, S. J., P. W. Clinton, R. B. Allen, and M. R. Davis. 2011. Climate cues and resources interact to determine seed production by a masting species. Journal of Ecology 99(3):870-877. https://doi.org/10.1111/j.1365-2745.2011.01803.x
Smith, A. N. H., and I. M. Westbrooke. 2004. Changes in bird conspicuousness at Pureora Forest. Notornis 51:21-25.
Spurr, E. B. 2000. Hen eggs poisoned with sodium monofluoroacetate (1080) for control of stoats (Mustela erminea) in New Zealand. New Zealand Journal of Zoology 27(3):165-172. https://doi.org/10.1080/03014223.2000.9518222
Spurr, E. B., and S. H. Anderson. 2004. Bird species diversity and abundance before and after eradication of possums and wallabies on Rangitoto Island, Hauraki Gulf, New Zealand. New Zealand Journal of Ecology 28(1):143-149. URL: http://www.jstor.org/stable/24058221
Vianen, V. J., O. R. Burge, A. T. MacFarlane, and D. Kelly. 2018. The effects of single aerial 1080 possum-control operations on common forest birds in the South Island, New Zealand. New Zealand Journal of Ecology 42(2):169-178. https://doi.org/10.20417/nzjecol.42.17
Walker, S., and A. Monks. 2018. Estimates of local occupancy for native land birds from the New Zealand bird atlases. Notornis 65(4):223-236.
Walker, S., A. Monks, and J. Innes. 2019. Thermal squeeze will exacerbate declines in New Zealand's endemic forest birds. Biological Conservation 237:166-174. https://doi.org/10.1016/j.biocon.2019.07.004
Whitau, K., D. Kelly, T. N. H. Galloway, A. E. T. MacFarlane, J. C. C. M. van Vianen, and K. Roberts. In press. Effects of altitude, seedfall and control operations on rat abundance in South Island Nothofagus forests 1998–2016. New Zealand Journal of Ecology.
Wilmshurst, J. M., A. J. Anderson, T. F. G. Higham, and T. H. Worthy. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences 105(22):7676-7680. https://doi.org/10.1073/pnas.0801507105
Wiser, S. K., P. J. Bellingham, and L. E. Burrows. 2001. Managing biodiversity information: development of New Zealand ’s National Vegetation Survey databank. New Zealand Journal of Ecology 25(2):1-17.
Wright, J. C. 2011. Evaluating the use of 1080: predators, poisons and silent forests. Parliamentary Commissioner for the Environment, Wellington, New Zealand. URL: https://www.pce.parliament.nz/media/1689/pce-1080-2017-web.pdf
Fig. 1

Fig. 1. Study site locations within Craigieburn Forest Park, Canterbury, New Zealand. Bird count stations were along tracks or gravel roads (plain thick lines) at three sites: Cheeseman (Ch), Broken River, with four subsites (Br - Broken River road, Bl - Lyndon track, Bd - Dracophyllum track, and Bm - Mistletoe track), and Craigieburn (Cr). Nothofagus solandri seedfall was measured at Broken River at 1,050 m elevation (dotted thick line). The base map was from New Zealand Topo50 maps.

Fig. 2

Fig. 2. Fitted mean (±SE) number for six common native bird species per 5-min bird count per seed year (where “1978” covers March 1978 to February 1979) in Craigieburn Forest Park; autumn (circle, solid line) and summer (triangle, dotted line). Fitted values are shown for Broken River, but the analysis uses data from all sites. When elevation was significant (Brown Creeper, Grey Warbler, and Silvereye), fitted values are predicted for 950 m elevation.

Fig. 3

Fig. 3. Fitted mean (±SE) number per 5-min bird count per seed year in Craigieburn Forest Park for three native bird species. There were no significant differences between seasons for Kea and Long-tailed Cuckoo. Codes and conventions as for Fig. 2. When elevation was significant (Fantail, Kea), fitted values are for 950 m elevation.

Fig. 4

Fig. 4. Fitted mean (±SE) number for six common exotic bird species per seed year in Craigieburn Forest Park. Conventions as for Fig. 2. Fitted values are for 950 m elevation when significant (Blackbird, Chaffinch, Redpoll, and Greenfinch).

Table 1
Table 1. Total number of 5-min bird counts by study, site/subsite, and season. The number of study years corresponds to calendar years. Sites were Cheeseman (Ch), Broken River with four subsites (Br - Broken River road, Bd - Dracophyllum track, Bl - Lyndon track, and Bm - Mistletoe track), and Craigieburn road (Cr). For analysis, counts were grouped into three seasons by including June counts in autumn and August counts in spring (southern hemisphere seasons).
| Study period | Years | Sites/subsites | Autumn (Mar–Jun) |
Spring (Aug–Nov) |
Summer (Dec–Feb) |
Total |
| 1978–1982 | 5 | Br, Bd, Bl, Bm | 3,680 | 2,720 | 1,920 | 8,320 |
| 1999–2004 | 6 | Br, Bd, Cr, Ch | 270 | 510 | 1,230 | 2,010 |
| 2019–2020 | 2 | Br, Bd, Bl, Cr, Ch | 278 | 0 | 330 | 608 |
| Total | 13 | 4,228 | 3,230 | 3,480 | 10,938 | |
Table 2
Table 2. Variation in Nothofagus solandri seedfall and pest control (stoat and wasp) at Craigieburn Forest Park. Seedfall (annual mean mountain beech seeds per m²) is given only for years with bird counts. Sites: Broken River = B, Cheeseman = Ch, and Craigieburn = Cr.
| Year | Seedfall/m² | Pest control | Pest control site | Reference |
| 1973–1977 | stoat | B | King (1983) | |
| 1978 | 593 | stoat | B | King (1983) |
| 1979 | 6,587 | |||
| 1980 | 8.1 | |||
| 1981 | 28.2 | |||
| 1982 | 6,600 | |||
| 1994 | stoat | B, Cr | Spurr (2000) | |
| 1999 | 6,083 | Kelly et al. (2005) | ||
| 2000 | 3,503 | stoat | B | Kelly et al. (2005) |
| 2001 | 13 | stoat | B | Kelly et al. (2005) |
| 2002 | 5,340 | Kelly et al. (2005) | ||
| 2003 | 4.9 | Kelly et al. (2005) | ||
| 2004 | 7,958 | Kelly et al. (2005) | ||
| 2007–2014 | stoat | B, Ch, Cr | Harris et al. (2022) | |
| 2015–2018 | stoat, wasp | B, Ch, Cr | Harris et al. (2022) | |
| 2019 | 5,355 | stoat, wasp | B, Ch, Cr | Harris et al. (2022) |
Table 3
Table 3. Raw mean birds per 5-min count for all birds recorded at Craigieburn across three study periods (1978–1982, 1999–2004, 2019–2020), ranked by overall mean abundance. *Long-tailed Cuckoo are migratory and present in New Zealand only for the breeding season (Summer), so their means are based only on summer counts.
| Bird common name | Species name | Status | 1978–1982 | 1999–2004 | 2019–2020 |
| Bellbird | Anthornis melanura | Native | 2.271 | 2.513 | 5.14 |
| Silvereye | Zosterops lateralis | Native | 1.345 | 0.877 | 1.613 |
| Chaffinch | Fringilla coelebs | Exotic | 1.243 | 0.825 | 1.286 |
| Common Redpoll | Carduelis flammea | Exotic | 1.749 | 0.641 | 0.729 |
| Rifleman | Acanthisitta chloris | Native | 1.062 | 0.347 | 0.507 |
| Tomtit | Petroica macrocephala | Native | 0.397 | 0.314 | 0.385 |
| Brown Creeper | Mohoua novaeseelandiae | Native | 0.407 | 0.288 | 0.064 |
| Grey Warbler | Gerygone igata | Native | 0.315 | 0.181 | 0.156 |
| Eurasian Blackbird | Turdus merula | Exotic | 0.163 | 0.105 | 0.089 |
| New Zealand Fantail | Rhipidura fuliginosa | Native | 0.061 | 0.119 | 0.039 |
| Dunnock | Prunella modularis | Exotic | 0.122 | 0.012 | 0.071 |
| European Greenfinch | Carduelis chloris | Exotic | 0.107 | 0.061 | 0.031 |
| European Goldfinch | Carduelis carduelis | Exotic | 0.056 | 0.003 | 0.11 |
| Kea | Nestor notabilis | Native | 0.038 | 0.08 | 0 |
| Song Thrush | Turdus philomelos | Exotic | 0.021 | 0.051 | 0.030 |
| Long-tailed Cuckoo * | Eudynamys taitensis | Native | 0.058 | 0.012 | 0.015 |
| Australasian Magpie | Gymnorhina tibicen | Exotic | 0.025 | 0.001 | 0 |
| Yellowhammer | Emberiza citrinella | Exotic | 0.008 | 0.002 | 0.013 |
| Parakeet spp. / Kakariki | Cyanoramphus spp. | Native | 0.0004 | 0.012 | 0.008 |
| Welcome Swallow | Hirundo neoxena | Native | 0 | 0 | 0.007 |
| New Zealand Falcon | Falco novaeseelandiae | Native | 0.001 | 0.001 | 0.002 |
| Swamp Harrier | Circus approximans | Native | 0.001 | 0 | 0.003 |
| Southern Black-backed Gull | Larus dominicanus | Native | 0.003 | 0 | 0 |
| Canada Goose | Branta canadensis | Exotic | 0 | 0 | 0.003 |
| Paradise Shelduck | Tadorna variegata | Native | 0 | 0.002 | 0 |
| Common Starling | Sturnus vulgaris | Exotic | 0.001 | 0 | 0 |
| Shag spp. | Phalacrocorax spp. | Native | 0.0005 | 0 | 0 |
| South Island Robin | Petroica australis | Native | 0 | 0.0005 | 0 |
| Eurasian Skylark | Alauda arvensis | Exotic | 0.0002 | 0 | 0 |
| Tui | Prosthemadera novaeseelandiae | Native | 0.0002 | 0 | 0 |
| New Zealand Pigeon | Hemiphaga novaeseelandiae | Native | 0.0001 | 0 | 0 |
| Mallard | Anas platyrhynchos | Exotic | 0.0001 | 0 | 0 |
Table 4
Table 4. Relationship between seedfall (annual mean number of beech seeds per m²) and number of birds per 5-min bird count for each bird species in Craigieburn Forest Park (see Append. 1: Table A1 for detailed formulas). Significant relationships in bold.
| Bird species | Estimate | Std. Error | z value | P |
| Native | ||||
| Bellbird | 0.033 | 0.010 | 3.365 | 0.001 |
| Rifleman | -0.012 | 0.012 | -1.078 | 0.281 |
| Brown Creeper | 0.025 | 0.020 | 1.232 | 0.218 |
| Tomtit | -0.013 | 0.016 | -0.791 | 0.429 |
| Grey Warbler | -0.031 | 0.023 | -1.353 | 0.176 |
| Silvereye | 0.008 | 0.019 | 0.446 | 0.656 |
| Fantail | -0.035 | 0.050 | -0.705 | 0.481 |
| Kea | 0.048 | 0.031 | 1.543 | 0.123 |
| Long-tailed Cuckoo | -0.063 | 0.081 | -0.776 | 0.438 |
| Exotic | ||||
| Blackbird | 0.103 | 0.028 | 3.673 | <0.001 |
| Chaffinch | 0.278 | 0.030 | 9.106 | <0.001 |
| Redpoll | 0.315 | 0.041 | 7.759 | <0.001 |
| Greenfinch | 0.374 | 0.059 | 6.396 | <0.001 |
| Goldfinch | 0.457 | 0.048 | 9.434 | <0.001 |
| Dunnock | 0.189 | 0.027 | 6.951 | <0.001 |
Table 5
Table 5. Long-term changes in counts for each study bird species at Craigieburn Forest Park based on models predicting number of birds per 5-min bird count from study period (1978–1982, 1999–2004, 2019–2020) and other covariables (see Append. 1: Tables A1, A2 for full models). P values are shown in brackets and “ns” means non-significant. * Brown Creeper: 2019–2020 significantly lower than 1978–1982 (z value = -2.022, P = 0.043); Grey Warbler: 2019–2020 significantly lower than 1978–1982 (z value = -3.028, P = 0.002). For full details of analyses see Append. 1: Table A2; for fitted means in each study period, see Append. 1: Table A4.
| Bird species | 1978–1982 to 1999–2004 | 1999–2004 to 2019–2020 |
| Native | ||
| Bellbird | Increase (0.017) | Increase (0.008) |
| Rifleman | Decrease (<0.001) | Increase (0.008) |
| Brown Creeper | ns (0.882) | Decrease* (0.065) |
| Tomtit | Decrease (0.013) | Increase (0.046) |
| Grey Warbler | Decrease (<0.001) | ns* (0.102) |
| Silvereye | ns (0.224) | ns (0.154) |
| Fantail | ns (0.837) | ns (0.718) |
| Kea | Increase (0.010) | ns (0.222) |
| Long-tailed Cuckoo | ns (0.120) | ns (0.732) |
| Exotic | ||
| Blackbird | ns (0.552) | ns (0.309) |
| Chaffinch | Decrease (0.013) | Increase (0.001) |
| Redpoll | ns (0.563) | ns (0.825) |
| Dunnock | Decrease (<0.001) | Increase (0.009) |
| Greenfinch | ns (0.704) | ns (0.705) |
| Goldfinch | ns (0.157) | Increase (0.044) |