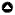The following is the established format for referencing this article:
Katuwal, H. B., K. S. G. Sundar, M. Zhang, B. Rimal, H. S. Baral, H. P. Sharma, P. Ghimire, A. C. Hughes, and R.-C. Quan. 2022. Factors affecting the breeding ecology of the globally threatened Lesser Adjutant (Leptoptilos javanicus) in agricultural landscapes of Nepal. Avian Conservation and Ecology 17(2):15.ABSTRACT
Many threatened birds use the mosaic of agricultural landscapes for foraging and breeding. Despite the reliance of many species on these habitats, few studies have investigated factors influencing the breeding ecology of storks in agricultural landscapes. We assessed site-level variables (tree height and location of nest tree; human habitation or non-human habitation), colony-level variables (colony size and chicks per nest), and landscape-level variables (area of human habitation, wetland area, and distance to the nearest wetland) to understand the factors influencing the breeding ecology of the globally threatened Lesser Adjutant (Leptoptilos javanicus) across multiple locations in the agricultural landscape of lowland Nepal during 2019–2020. We monitored 65 active colonies that had 206 active nests in five study sites. Two hundred eighty chicks fledged from these colonies, with 13% (n = 41) chick mortality. Most colonies were in agricultural land (51%) and human habitation (28%). Lesser Adjutant colonies located on tall trees such as Bombax ceiba (57%), Haldina cordifolia (11%), and Ficus religiosa (11%); however, these tree species were used much more than their availability on the landscape. Tree height had a significant positive influence on colony site selection and colony size, whereas colony size positively influenced fledgling success. Measured landscape variables did not have significant relationships with breeding success metrics. The agricultural landscapes of lowland Nepal provided important breeding habitat for Lesser Adjutants, and the suitability of sites with colonies related more to site-level and colony-level than landscape-level variables. Increasing urban development of agricultural landscapes is likely the greatest threat to breeding Lesser Adjutants, with the decline of suitable nesting trees being a potential additional threat. Lowland Nepal's agricultural landscapes support significant breeding populations of Lesser Adjutants that had considerable breeding success, underscoring the urgent need to support traditional agriculture that favors large waterbirds.RÉSUMÉ
INTRODUCTION
Many bird species are declining more rapidly in agricultural landscapes than other land use types (Stanton et al. 2018, PECBMS 2020). The major reasons for these declines are urbanization, hunting and trade, chemical poisoning, electrocution, agricultural intensification, lack of suitable nesting trees, and varying rainfall patterns or temperature anomalies associated with global climate change (Inskipp and Baral 2010, Mitra et al. 2011, Pearce-Higgins et al. 2015, BirdLife International 2022, Katuwal et al. 2021, 2022). Significant declines have been noticed in the global north, where farmers practice large-scale monoculture agriculture with extensive mechanization and chemical use (Stanton et al. 2018, Reif and Vermouzek 2019). There has been relatively little research on species diversity and changes in their abundance in agricultural areas in developing countries. This paucity of research has prevented an understanding of the impacts of agriculture on bird populations in these areas, and there is little detailed information on the factors that influence the suitability of specific landscapes for individual bird species (King et al. 2010).
Agricultural landscapes in developing countries are often heterogeneous because of smaller land holdings (< 2 ha per farmer; Lowder et al. 2016, Ricciardi et al. 2021). The diversity of landscape conditions is due to farmers retaining patches of natural habitats such as wetlands, shrublands, grasslands, and a variety of tree species as patches or scattered single trees (Koju et al. 2019, Sundar et al. 2019, Kittur and Sundar 2021). In South Asia, traditional agricultural practices often include traditional agroforestry (planting and retaining trees) and preservation of common lands (e.g., wetlands; Inskipp and Baral 2010, Sundar and Kittur 2012, Amatya et al. 2018, Kittur and Sundar 2021). Remnant habitat patches in these landscapes has improved landscape-level occupancy of a diverse assemblage of resident birds at landscapes scales (Sundar and Kittur 2012) and has facilitated relatively high breeding success of several large waterbird species (Sundar 2011, Kittur and Sundar 2021).
The growing number of studies in cereal-dominated, multi-cropped landscapes has focused on large waterbirds. For some waterbirds, such as Sarus Cranes (Grus antigone), retaining wetlands in croplands with three to five crops per year improved breeding success relative to crane pairs whose territories had only rice paddies (Sundar 2009). Conversion of croplands and wetlands to urban areas or human habitations permanently removed breeding pairs, reducing the breeding population (Sundar 2011). Black-necked Storks (Ephippiorhynchus asiaticus) also benefitted from the presence of wetlands but tolerated some conversion to urban areas (though they were strongly affected by changing rainfall patterns; Sundar 2011). In contrast, Asian Woolly-necks (Ciconia episcopus) situated nests close to irrigation canals in scattered trees planted far from human habitation and wetlands (Kittur and Sundar 2021). These few available studies have underscored the importance of persisting natural features and artificial structures such as canals introduced to benefit farmers in sustaining impressive populations of breeding large waterbirds on South Asian agricultural landscapes.
The Lesser Adjutant (Leptoptilos javanicus) is another large waterbird species (Fig. 1) that was, until recently, assumed to require undisturbed forests and wetlands for its breeding (Poudyal and Nepal 2010, Inskipp et al. 2016, BirdLife International 2021, Bhattarai et al. 2021). Relatively few studies have been conducted on the ecology of this species (Table 1). The majority of these studies have been anecdotal observations, with a small but growing number of studies led by a priori hypotheses (Table 1). Lesser Adjutants in Nepal represent a small portion of the species’ breeding range, yet most research on the species has been conducted in Nepal (Table 1). Lesser Adjutants are usually distributed and breed in lowland Nepal (< 300 m elevation; Inskipp et al. 2016). The largest known breeding population has been discovered in the agricultural matrix of central lowland Nepal (Sundar et al. 2016). Most stork colonies were on trees planted for multifunctional agroforestry, and storks selected the largest trees available on the landscape for nesting (Koju et al. 2019). Breeding success was influenced by the interaction of various factors, such as colony size, wetland area, human habitation, and changing crops (Sundar et al. 2019). This suggests that variables at multiple spatial scales work together on Lesser Adjutant colonies located in agricultural landscapes to affect overall breeding success. However, it is not clear if findings from one location are transferable to the range of conditions that this species breeds in across lowland Nepal’s landscape. Do Lesser Adjutants favor larger forest tree species in areas with more forests? Are colony locations and breeding success related to site-level, colony-level, and landscape-level variables similarly across lowland Nepal? Understanding the answers to these questions is critical because this species is globally and nationally Vulnerable (Inskipp et al. 2016, BirdLife International 2021).
In this study, we investigated the factors affecting the location of colonies and breeding success of Lesser Adjutants in multiple locations across lowland Nepal, where smallholder agriculture is dominant (Fig. 2). We used several variables measured at site, colony, and landscape scales, including some that have been measured in past studies (Table 1). We then analyzed the impact of these variables to understand whether Lesser Adjutant breeding was (1) influenced more by site-level, colony-level, or landscape-level variables, and (2) whether these variables influenced colony site selection and breeding success similarly in multiple locations.
METHODS
Study area
We conducted this study in multiple locations across southern lowland parts (< 300 m elevation) of Nepal (Fig. 2). This landscape primarily consists of floodplains of different river systems and is largely suitable for agriculture. Multiple crops are grown in each field over the year, with flooded rice paddies dominating during the monsoon or rainy season (June–September), and wheat, maize, sugarcane, and lentils during the winter (November–February). The fields are kept largely fallow during the hot summers (March–June; Koju et al. 2019, Katuwal et al. 2020). Lowland Nepal’s climate is a mix of tropical and sub-tropical types. The monthly average rainfall for 2015–2019 in the study areas ranges from 80–155 mm and the minimum and maximum temperature for the period was from 18–33 °C, respectively (DHM 2021). Thick fog for a few weeks as well as cold and rain for a few days in December and January are characteristic of the winter season.
The landscape of lowland Nepal is primarily agricultural. However, traditional farming methods are practiced that are characterized by relatively low mechanization, trees retained amid crop fields and along the roads and canals, and retention of community-use wetlands of various sizes on the landscape (Koju et al. 2019). Wetlands are retained primarily for use by humans and livestock, and they experience year-long use. Trees are retained for multifunctional agroforestry with farmers growing some species for silviculture (e.g., Bombax ceiba), retaining other species for spiritual reasons (e.g., Ficus religiosa), and maintaining commercially valuable fruit-bearing species as both individual scattered trees and as groves (e.g., Mangifera indica; Koju et al. 2019). Human habitation is restricted to relatively high-density villages, towns, and cities; however, scattered houses or small brick factories have been increasing in agricultural lands in recent years (Katuwal et al. 2022), and human presence occurs in the crop fields throughout the year (Koju et al. 2019). Fields are both rain fed and irrigated by a network of canals that has been increasing in recent years. Irrigation canals lead to the formation a very large number of small wetlands whose hydrology is linked to flooding patterns of the canals (H. B. Katuwal and K. S. Gopi Sundar, personal observations). Forest patches are largely preserved as community forests or protected reserves, and the extent of forests in lowland districts varies greatly (Rimal et al. 2018a, MFE 2019). Landholdings are relatively small (< 1 ha; Katuwal et al. 2021), but farmers retain many very small patches of natural grasslands, degraded scrub, bamboo, and open uncultivated areas interspersed with croplands. Therefore, the overall landscape is diverse and patterned with a relatively high and densely populated human presence throughout the year, but it retains natural features alongside croplands and artificial features, such as canals built to support farming (Katuwal et al. 2022).
Research design
We focused on understanding the breeding ecology of the Lesser Adjutant across lowland Nepal in areas that had different levels of agriculture, forests, wetlands, and human population. We initially included seven different regions for surveys (Fig. 2). Searches for stork colonies, however, located none in Chitwan (excluding core areas of Chitwan National Park) and Kailali districts. We retained the other five regions (Kapilvastu-Rupandehi, Bara, Sarlahi, Udayapur and Morang-Jhapa) for this study (Fig. 2).
Identifying and monitoring nesting colonies
Based on existing information and knowledge gleaned from local experts in each location, we selected parts of the focal districts for detailed surveys to locate Lesser Adjutant colonies. Two authors (H. B. Katuwal and K. S. Gopi Sundar) trained research associates at each site to locate and monitor colonies, observe fates of individual nests, and obtain measurements of trees (both nest trees and those measured to assess availability).
The nest initiation period of Lesser Adjutants in lowland Nepal is from August to October, with chicks remaining in colonies until they fledge in February (Sundar et al. 2016). Therefore, each focal study area was surveyed starting from 15 July 2019, following methods previously used in Nepal to study Lesser Adjutants’ breeding biology (Sundar et al. 2016, Koju et al. 2019). Motorable road routes were traversed using motorbikes, farmers and local researchers were interviewed, and locations mentioned in previous publications were visited to locate stork colonies. A colony was defined as having at least one chick in a nest or set of nests on trees whose canopies were contiguous, following previous studies (Sundar et al. 2016). At each colony, we recorded its location using a handheld Garmin Global Positioning System (GPSMAP 64s), identified the nest tree species, and measured the nest tree height in meters using a range finder (MILESSEY S9) and diameter at breast height (DBH) in centimeters.
Each colony was visited multiple times (1–2 times per month) until January 2020, when the last remaining chicks fledged. We observed nesting materials being collected from 16 July. We noted the first chick on 18 September (the chick might have hatched before but was not visible from the ground) and noticed the first chick fledging on 27 November. Colony size was the maximum number of nests observed at each colony. Lesser Adjutant pairs were not observed using nests of another pair, as in other stork species such as the White Storks (Ciconia ciconia; Vergara et al. 2006). This made it easy to estimate the sizes of all colonies definitively. At each colony, we recorded the number of chicks that fledged from each nest. Lesser Adjutants’ pre-fledged chicks were not observed moving around the colony or using nests of multiple pairs as had been observed in other stork species such as Painted Storks (Mycteria leucocephala; K.S. Gopi Sundar, personal observation). Therefore, counts of surviving and fledged chicks per nest were relatively straightforward. We did not climb trees to observe nests; therefore, we are unable to provide information on clutch size, hatching success (proportion of eggs that successfully hatched), and total breeding success (proportion of eggs and hatched chicks that successfully fledged). In addition to systematically monitored colonies, we recorded 13 additional colonies in other districts on an ad hoc basis until March 2020 (Appendix 1, Fig. A1.1) but did not include those for final analyses because the data sets were not comparable.
Measuring tree availability
We generated random points (for four locations) and systematically located points (Kapilvastu-Rupandehi area) using ArcGIS 10.4 to understand the tree availability for the stork to nest in the study area (Fig. 2). All accessible points were visited and the nearest trees’ species name, height, and DBH were recorded. We measured a total of 298 trees of 38 species to determine tree availability.
Mapping land use
We acquired terrain-corrected 30 m resolution satellite images from Landsat–8 OLI (10 November to 5 December 2019) to determine land use from the United States Geological Survey (https://earthexplorer.usgs.gov) for each focal study area within three sections, Kapilvast-Chitwan, Bara-Sarlahi, and Udayapur-Jhapa. The geometric accuracy of all collected images was verified. This process was conducted one image at a time, registered, and projected in Universal Transverse Mercator zone 45N. We used the ENVI v5.3 environment for processing all the images (https://www.l3harrisgeospatial.com/). We converted the digital number of images into radiance and applied the flash line-of-sight atmospheric analysis of spectral hypercubes (FLAASH) atmospheric correction model (Rimal et al. 2018b). We extracted land-use and land-cover variables using a support vector machine (Kavzoglu and Colkesen 2009, Schneider 2012) and radial basis function with assigned maximum penalty parameters to value 100 following Rimal et al. (2019). We classified land use in images into eight classes, as follows: urban (all built-up areas), agriculture land, forest (all forest types), shrubland, barren land (cliffs, small landslide, bare rocks, and unused lands), sand, water bodies, and grasslands. Overall accuracy was 91% for Kapilvastu-Chitwan, 87% for Bara-Sarlahi, and 90% for Udayapur-Jhapa sections.
Around each colony, using classified satellite imageries, we measured the amount of farmlands and wetlands (in ha) using a buffer of 5 km radius, assuming that storks would use these areas for foraging and that measurements at this scale could influence both colony location or site and breeding success (see Sundar et al. 2019). We also measured human habitation (built-up areas in ha) around each colony using a buffer of 1 km radius to assess whether human presence influenced both colony location and breeding success. We also calculated the nearest distance between colonies’ locations with land uses (patches > 0.25 ha) such as human habitation, wetland, and farmland. We used these three land-use classes because they have been seen to influence stork breeding (Tourenq et al. 2004, Onmuş et al. 2012, Koju et al. 2019, Sundar et al. 2019).
Data analysis
We assessed tree species selection by Lesser Adjutants with the use-availability framework of Manley et al. (2004), whereby selection metrics were contrasted against Bonferroni’s 95% confidence intervals to assess whether a particular species of tree was selected (used in higher proportion relative to availability), avoided (used less relative to availability), or used in similar proportion to availability. We used the WidesI function in the adehabitat package for estimations (Calenge 2006). Using the Wilcoxon rank-sum test (W) and ridgeline density plots in the ggridges package, we compared tree size (height and DBH) of those with colonies and those measured at systematic or random locations to determine whether Lesser Adjutants selected trees of specific sizes to locate colonies (Wilke 2021).
We calculated the mean (± SD) of several colony-level breeding variables including colony size, total number of chicks, chicks per nest, and fledgling success per colony. We estimated chicks per nest by dividing the total number of chicks by the number of nests or colony size in each colony. We considered fledgling success, i.e., the number of chicks fledged per colony, as the proxy for breeding success because it incorporates egg and chick mortality (see Sundar et al. 2019). We confirmed chick mortality by direct observations where possible and asked local people living near the colonies when we could not confirm mortality incidents directly. We performed pairwise Pearson correlation tests of the selected land-use variables and tree height and DBH, and subsequently removed redundant variables. For analysis, we retained tree height, wetland area and human habitations area around colonies, and distance to the wetland from the colony.
We were interested to understand the relative importance of site-level variables measured at the scale of the colony (tree height, location of nest tree; human habitation or non-human habitation) and at the scale of the larger landscape around the colony (distance to nearest wetland, area of human habitation, and wetland areas). We were interested in assessing the relative importance of these variables in influencing three discrete aspects of breeding, as follows: (1) nest/colony site selection, (2) colony size, and (3) fledgling success. These three aspects parsed out disparate and critical components of the breeding biology of Lesser Adjutants and, collectively, would allow identification of variables important to each stage of breeding (see also Sundar et al. 2016, Koju et al. 2019).
We checked for spatial autocorrelations using Moran’s I test in the ape package (Paradis and Schliep 2019) and found no significant spatial autocorrelation for all dependent variables (P > 0.05). We used a generalized linear mixed model (GLMM) to understand which variables influenced colony site selection and colony size. We further added colony-level variables (e.g., colony size and chicks per nest) to identify variables influencing fledgling success. To understand colony site selection, we used 65 random points (equalling the number of colonies) as colony/nest absence from 298 random surveys done to measure tree availability. We used tree height and extracted landscape variables of these points. Therefore, our response variables were, (1) colony site selection (presence/absence), (2) colony size (count), and (3) fledgling success (count). Similarly, our predictors were, site-level (tree height and location of nest tree; human habitation or non-human habitation), colony-level (colony size and chicks per nest), and landscape-level (area of human habitation and wetland area, and distance to the nearest wetland).
In our GLMM analysis, we assigned family binomial distribution for colony site selection and Poisson distribution for colony size and fledgling success. We included study site as the random effect in all models. We checked for overdispersion of our three models using overdisp_fun and found no overdispersion. Then, we used an information-theoretic approach (Burnham and Anderson 2002) and reported model averaging with delta corrected Akaike Information Criteria (ΔAICc) < 4 using the package MuMIn (Barton 2020). We calculated standardized coefficients of each model and considered the importance of each variable as high or significant when its 95% confidence interval (CI) did not overlap with zero (Di Stefano 2004). Because two of our study sites (Kapilvastu-Rupandehi and Morang-Jhapa) had colonies dispersed (≥ 20 colonies in each site) more than the other three areas, we reran the analysis to assess whether factors affecting breeding ecology would be consistent with these two sites. All analysis was performed in R software (R Core Team 2019).
RESULTS
We monitored 65 active colonies with 206 active nests of Lesser Adjutant until chicks fledged (Fig. 2). The majority of the colonies were located on agricultural lands (50.8%, n = 33), followed by human habitations (27.7%, n = 18) and shrub/grassland patches (16.9%, n = 11), whereas 4.6% (n = 3) of colonies were in forests. The mean (± SD) distance between colonies and agricultural lands was 54.5 m ± 87.5 (range: 0–464 m), human habitation was 150.1 m ± 173.7 (range: 0–752 m), and wetland was 862.1 m ± 772.4 (range: 61–3390 m).
Details of breeding colonies
From the 206 active nests located, 280 chicks fledged. The mean (± SD) colony size was 3.2 ± 2.3 (range: 1–15), total chicks was 4.9 ± 3.8 (range: 1–27), chicks per nest was 1.6 ± 0.44 (range: 1–3), and fledgling success was 4.3 ± 3.7 (range: 0–24). We recorded a higher number of colonies in the Kapilvastu-Rupandehi area (n = 29), followed by Morang-Jhapa (n = 20) and Bara (n = 8); Udayapur and Sarlahi had the fewest colonies (n = 4; Fig. 2). However, the mean colony size was higher in Sarlahi (mean = 6.25), followed by Udayapur and Bara (mean = 3.75), whereas the lowest was in Morang-Jhapa (mean = 2.8) and Kapilvastu-Rupandehi (mean = 2.7). There was 13% (n = 41) chick mortality, possibly due to extreme weather events (continuous rain, cold temperatures, or wind) that caused chicks to fall from nests (n = 9), tree felling by villagers (n = 7), monkeys (n = 4), hunting (n = 2), and unknown reasons (n = 19). Additionally, we recorded 90 chicks in 54 nests in 13 opportunistically recorded colonies (Appendix 1, Fig. A1.1). Altogether, 370 chicks fledged from 260 nests of 78 active colonies, accounting for 890 Lesser Adjutants (520 breeding individuals and 370 fledged chicks) in lowland Nepal.
Tree characteristics of the nesting and available trees
Lesser Adjutants nested on nine tree species out of 38 species located during visits to random points (23.6%). Tree species used versus those available varied across study sites. Albizia lucidior, Bombax ceiba, Haldinia cordifolia, Terminalia bellerica, Ficus religiosa, Shorea robusta, and Trewia nudiflora were used more than were available (Table 2). Bombax ceiba (56.9%, n = 37) had the most colonies, followed by Haldina cordifolia (10.8%, n = 7), Ficus religiosa (10.8%, n = 7), and Trewia nudiflora (7.7%, n = 5). Other trees had fewer colonies (n = 1–3; Table 2). However, the most commonly available trees were Dalbergia sissoo (21.8%, n = 65), Breonia chinensis (14.4%, n = 43), Eucalyptus spp. (14.1%, n = 42), Mangifera indica (12.8%, n = 38), and Bombax ceiba (8.1%, n = 24).
Trees used for nesting by storks varied significantly from available trees in both height (W = 5225, P < 0.001) and DBH (W = 2384.5, P < 0.001). The mean (± SD) height of nest trees (17.07 m ± 5.66) was ~ 5 m more than available trees (12.76 m ± 8.39), whereas DBH of nest trees (98 cm ± 37.78) was twice that of available trees (45.61 cm ± 27.72). These differences were visible at each study site (Fig. 3a and 3b). In addition, the height and DBH of the nesting tree with most colonies (Bombax ceiba) also varied within the study area (Fig. 3c and 3d).
Factors influencing breeding ecology
The effects of site-level and colony-level variables were more influential than landscape-level variables on the breeding of the Lesser Adjutant in Nepal (Fig. 4). Tree height had significant positive (95% CI did not overlap with zero) impacts on the colony site selection (Fig. 4a). Location of nest tree (non-human habitation negatively and human habitation positively) also had significant impacts on the colony site selection (Fig. 4a). Tree height also had a significant positive (95% CI did not overlap with zero) influence on the colony size (Fig. 4b). However, colony size had a higher significant positive (95% CI did not with overlap zero) influence on fledgling success relative to other variables (Fig. 4c). The landscape variables did not significantly impact breeding metrics (95% CI overlapped with zero). Notwithstanding statistical significance, the area of human habitations had a slightly negative relationship, whereas the distance to wetland and wetland area had positive relationships to breeding metrics (Fig. 4).
For study sites analyzed separately, results were identical with colony site selection and fledgling success. However, colony size had a significant negative relationship with the area of human habitation, whereas tree height had significant positive impacts (95% CI did not overlap with zero; Appendix 1, Fig. A1.2).
DISCUSSION
This is the first study of Lesser Adjutant breeding ecology, where colonies at multiple sites across lowland Nepal were studied simultaneously. Our study provides insights into the importance of nest tree species and height- and colony-level variables on the breeding of the Lesser Adjutant. Our study further demonstrates the value of Nepal’s agricultural landscapes for breeding Lesser Adjutants that were incorrectly assumed to require forests and protected wetlands (Poudyal and Nepal 2010, Inskipp et al. 2016, Bhattarai et al. 2021). We were able to confirm that the largest breeding populations are in central Nepal, where previous studies were conducted (see Sundar et al. 2019, Koju et al. 2020). Furthermore, we discovered that other sites in lowland Nepal also have breeding Lesser Adjutants, and the new sites collectively add up to significant breeding populations.
In our study, the drivers of mortality (13%) varied by study sites and included, for example, extreme weather events (continuous rain and cold for weeks or wind) in Udayapur (S. Chaudhary, personal communication), tree felling in Kapilvastu-Rupandehi, and hunting in Morang-Jhapa and eastern Nepal, in addition to a variety of unknown causes. In other studies, Large-billed Crows (Corvus macrorhynchos) were identified as a threat (south-east Asia; Clements et al. 2013), though nest failures did not occur during other studies (see Sundar et al. 2016). Hunting of the species has been recorded in east Nepal (S. Thapa and A. Sah, personal communication); however, the impact of tree felling is rarely documented. Clearly, inter-annual and inter-site variations of chick mortalities exist for Lesser Adjutants in Nepal. The fledgling success reported in our study is slightly lower than Sundar et al. (2016) reported at Kapilvastu-Rupandehi, which was also the site that provided the largest sample size for our study. However, fledgling success in our study is higher than that reported by Karki and Thapa (2013) in eastern Nepal, which had a much smaller sample size. These variations are likely driven by sample sizes, although inter-annual variations in breeding success cannot be ruled out.
We did not find colonies in agricultural lands along the west of Kapilvastu-Rupandehi, showing that Lesser Adjutants may have a lower population in the region and may predominantly breed in protected forested parks (Sharma 2006; D. Joshi, personal communication). The total population of Lesser Adjutants in this study was 890, which, along with the scale of the study, is the highest count of the species in Nepal. Our surveys did not cover the entirety of the known breeding distribution range in Nepal, suggesting that the actual breeding population is higher. Because of site-level variations in breeding populations, we are unable to provide a robust extrapolation but suspect that the population is much higher than the < 1000 individuals guesstimated for Nepal (Inskipp et al. 2016).
In our study, tree species and size drove the nest site selection of Lesser Adjutant. Storks had most colonies on Bombax ceiba at all sites, although Haldina cordifolia and Ficus religiosa were also widely used. Retaining these tree species on the landscape for longer periods is essential to provide nesting sites for breeding Lesser Adjutants. A large number of available trees of these species were much smaller than nest trees, suggesting that agroforestry practices that favor larger trees will be beneficial for Lesser Adjutant Storks in the region. Other threats to nest trees were development projects, thunderstorms (two nesting trees fell in our study because of storms), and, very rarely, people removing nest trees in response to the nuisance of nesting birds (G. Sah, personal communication). Lesser Adjutants used native tree species almost entirely and completely avoided the much commonly available exotic Eucalyptus sp. Asian Woolly-necks in India used both native trees and the exotic Eucalyptus sp., suggesting that each stork species has nuanced and idiosyncratic preferences for nest trees (Kittur and Sunder 2021). Taller trees were important for locating colonies, as observed in other stork studies (Hilaluddin et al. 2003, Koju et al. 2019). Such a choice likely reduces human-related disturbance and storm-related tree uprooting. Lesser Adjutants made more choices among nest tree characteristics and fewer choices among location of the trees, which were frequently in human settlements.
Although colony-level variables, such as colony size, have been documented to influence breeding success (Serrano et al. 2005, Sundar et al. 2019) and provisioning behavior (Sundar et al. 2016), few studies only include these variables when identifying the factors affecting large waterbird breeding success. Our studies of Lesser Adjutants in Nepal underscore the need to include colony-level variables. This may be especially true for species that do not have very large colonies because colony-level variables likely influence breeding behaviour. (Minias et al. 2020).
Lesser Adjutants were not influenced greatly by landscape-level variables relative to site-level and colony-level variables. This finding is different from other studies that have shown similar landscape-level variables to have either positive or negative influences on breeding success of waterbirds (Sundar 2011, Janiszewski et al. 2014, Kittur and Sundar 2021). We did note a slight negative influence of area of human habitations on both colony size and fledgling success. However, some colonies were observed within human habitations, possibly because of lack of availability of large trees in agricultural habitats. Kittur and Sundar (2021) showed single-nesting Asian Woolly-necks avoiding human habitations for nesting. Overall, it is possible that the Lesser Adjutants, which have relatively small colony sizes, will be negatively impacted by increased urbanization in Nepal.
Contrary to our predictions, wetland areas around colonies and distance to wetlands weakly influenced breeding metrics. It is not immediately clear why storks were not associated more strongly with wetlands on lowland Nepal. Previous studies have underscored the strong relationship between foraging activity and changing crops, suggesting that crop fields are important foraging habitats for Lesser Adjutants and that the dominant crop fields may exceed the positive influence of wetlands alone (Sundar et al. 2016). It is also possible that the current wetland extent and the number of wetlands on Nepal’s agricultural landscapes are high enough to not strongly influence stork breeding. Wetland’s influence on the Lesser Adjutants may increase in strength if wetland deterioration continues but is an aspect that requires longer term monitoring.
CONCLUSIONS
Our study shows that agricultural landscapes with multifunctional agriculture are vital to support Lesser Adjutant breeding populations. We have identified variables that are important for each stage of breeding; all of these variables require conservation focus to enable continued breeding success of Lesser Adjutants. Larger trees influenced colony location and, in turn, supported larger colonies will have a greater breeding success. Nepal’s governmental policy currently does not include maintaining agricultural landscapes for wildlife conservation, and wildlife research in such areas is minimal. Our work demonstrates the need to increase important nest trees and protect existing large trees. Lesser Adjutants in Nepal exemplify how agriculture and wildlife can coexist. This underscores the need to explore additional South Asian farmlands to document similar, potentially novel, situations that enable biodiversity conservation amid cultivation.
RESPONSES TO THIS ARTICLE
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a response, follow this link. To read responses already accepted, follow this link.ACKNOWLEDGMENTS
This work has been supported by CAS-SEABRI (Y4ZK111B01) program. HBK would like to thank CAS-TWAS President's Fellowship for providing the PhD fellowship. We thank the Department of Forests and Soil Conservation and the Department of National Parks and Wildlife Conservation, Nepal for providing permissions to carry out this work in Nepal. We would like to thank Nature Conservation Foundation for administrative support and Swati Kittur, Kailash Jaiswal, Yam Mahato, Prashant Rokka, Sabal Sharma, Sachet-Anand Chaudhary, Netra Koirala, Anis Timsina, Sandip Luitel, Ganesh Tamang, Asmit Limbu, Shailendra Yadav, Sabin Adhikari, Sandhya Sharma, Jeevan Rai, Dev Narayan Mandal, Deven Kharel, Tapil Rai, Bibek Gautam, Nabin Pandey, Bibek Belbase, Aarati Basnet, Santosh Bajagain, Shantosh Bhattarai, Raj Kumar Rai, Sanjan Thapa, Aklesh Sah, and Ruchuan He for their help, cooperation, and sharing information during the project period.
LITERATURE CITED
Amatya, S. M., E. Cadamon, and I. Nuberg. 2018. Agroforestry systems and practices in Nepal. Revised edition. Agriculture and Forestry University, Rampur, Nepal.
Bajagain, S., and A. Pradhan. 2018. Note on nesting behavior of globally threatened Lesser Adjutant Leptoptilos javanicus in Sarlahi, Nepal. Danphe 27:9-10.
Bajagain, S., A. Pradhan, and A. Bhusal. 2019. Confirmation of breeding colonies of Lesser Adjutant Stork in Sarlahi, Nepal. Himalayan Naturalist 2:34-36.
Bandara, A. M. R. S., and K. B. Ranawana. 2014. A preliminary survey on Lesser Adjutant (Leptoptilos javanicus) in Kumana National Park, Eastern Province Sri Lanka. Wildlanka 2:59-63.
Baral, B., S. Bhandari, S. Koirala, P. Bhandari, G. Magar, D. R. Basnet, J. Rai, and H. S. Baral. 2020. First distributional record of the Lesser Adjutant Leptoptilos javanicus Horsfield, 1821 (Ciconiiformes: Ciconiidae) from Sindhuli District, Nepal. Journal of Threatened Taxa 12:17028-17031. https://doi.org/10.11609/jott.4902.12.14.17028-17031
Bartoń, K. 2020. MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn
Bhattarai, B. P., J. N. Adhikari, and M. Rijal. 2021. Nesting habitat selection and challenges of conservation of the vulnerable Lesser Adjutant Leptoptilos javanicus (Horsfield, 1821) in the Chitwan National Park, Nepal. Ornis Hungarica 29:33-46.
BirdLife International. 2021. Species factsheet: Leptoptilos javanicus. http://datazone.birdlife.org/species/factsheet/lesser-adjutant-leptoptilos-javanicus
BirdLife International. 2022. State of the world’s birds: 2020 annual update. http://datazone.birdlife.org/2020-annual-update?fbclid=IwAR0ndxaHyCJompEUMcGHgJV8__2XGFlHnO3mWUtNSVvhRSRUjtjseKwKzCw
Burnham, K. P., and D. R. Anderson. 2002. Model selection and inference: a practical information-theoretic approach. Springer, New York, New York, USA.
Calenge, C. 2006. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecological Modelling 197:516-519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Choudhury, A. 2005. First record of Lesser Adjutant Leptoptilos javanicus for Bhutan. Forktail 21:164-165.
Chowdhury, S. U., and M. S. H. Sourav. 2012. Discovery of Lesser Adjutant Leptoptilos javanicus breeding colony in Bangladesh. BirdingASIA 17:57-59.
Clements, T., H. Rainey, D. An, V. Rours, S. Tan, S. Thong, W. J. Sutherland, and E. J. Milner-Gulland. 2013. An evaluation of the effectiveness of a direct payment for biodiversity conservation: The Bird Nest Protection Program in the Northern Plains of Cambodia. Biological Conservation 157:50-59. https://doi.org/10.1016/j.biocon.2012.07.020
Department of Hydrology and Meteorology (DHM). 2021. Meteorological data of Nepal. DHM, Kathmandu, Nepal. http://www.hydrology.gov.np/#/?_k=vek2br
de Silva, T. N., S. Fernando, H. de Silva, and P. Tennakoon. 2015. Lesser Adjutant Leptoptilos javanicus Horsfield, 1821 (Ciconiiformes: Ciconiidae) in the dry lowlands of Sri Lanka: distribution, ecology and threats. Journal of Threatened Taxa 7:8089-8095. https://doi.org/10.11609/jott.2425.7.14.8089-8095
Di Stefano, J. 2004. A confidence interval approach to data analysis. Forest Ecology and Management 187:173-183. https://doi.org/10.1016/S0378-1127(03)00331-1
Dwivedi, M. D., S. Prakash, A. K. Mishra, V. Prakash, G. Dwivedi, and M. Raziuddin. 2013. First breeding record of the Lesser Adjutant Leptoptilus javanicus (Horsfield, 1821) from Bokaro district, Jharkhand, India. Journal of the Bombay Natural History Society 110:222-223.
Gopi, G. V, and B. Pandav. 2007. Observations on breeding biology of three stork species in Bhitarkanika mangroves, India. Indian Birds 3:45-50. http://indianbirds.in/pdfs/IB.3.2.45-50.pdf
Hilaluddin, J. N. Shah, and T. A. Shawl. 2003. Nest site selection and breeding success by Cattle Egret and Little Egret in Amroha, Uttar Pradesh, India. Waterbirds 26:444-448. https://doi.org/10.1675/1524-4695(2003)026[0444:NSSABS]2.0.CO;2
Inskipp, C., and H. S. Baral. 2010. Potential impacts of agriculture on Nepal birds. Our Nature 8:270-312. https://doi.org/10.3126/on.v8i1.4339
Inskipp, C., H. S. Baral, S. Phuyal, T. R. Bhatt, M. Khatiwada, T. Inskipp, A. P. Khatiwada, S. Gurung, P. B. Singh, L. Murray, L. P. Poudyal, and R. Amin. 2016. The status of Nepal’s birds: The national red list series. Zoological Society of London, United Kingdom. https://www.zsl.org/conservation/regions/asia/national-red-lists-of-nepals-birds-and-mammals
Janiszewski, T., P. Minias, Z. Wojciechowski, and P. Podlaszczuk. 2014. Habitat selection by White Storks breeding in a mosaic agricultural landscape of Central Poland. Wilson Journal of Ornithology 126:591-599. https://doi.org/10.1676/13-219.1
Karki, S., and T. B. Thapa. 2013. Population status, nesting habitat selection and conservation threats of Lesser Adjutant Stork (Leptoptilos javanicus) in the eastern lowlands of Nepal. Conservation Science 1:27-35. https://doi.org/10.3126/cs.v1i1.8581
Katuwal, H. B., H. S. Baral, H. P. Sharma, and R.-C. Quan. 2020. Asian Woollynecks are uncommon on the farmlands of lowland Nepal. SIS Conservation 2:50-54. https://storkibisspoonbill.org/wp-content/uploads/2021/04/2020Katuwal_Final.pdf
Katuwal, H. B., J. Rai, K. Tomlinson, B. Rimal, H. P. Sharma, H. S. Baral, A. C. Hughes, and R.-C. Quan. 2022. Seasonal variation and crop diversity shape the composition of bird communities in agricultural landscapes in Nepal. Agriculture, Ecosystems & Environment 333: 107973. https://doi.org/10.1016/j.agee.2022.107973
Katuwal, H. B., M. Zhang, H. S. Baral, H. P. Sharma, and R.-C. Quan. 2021. Assessment of farmers’ knowledge and perceptions towards farmland birds show the need of conservation interventions. Global Ecology and Conservation 27:e01563. https://doi.org/10.1016/j.gecco.2021.e01563
Kavzoglu, T., and I. Colkesen. 2009. A kernel functions analysis for support vector machines for land cover classification. International Journal of Applied Earth Observation and Geoinformation 11:352-359. https://doi.org/10.1016/j.jag.2009.06.002
King, S., C. S. Elphick, D. Guadagnin, O. Taft, and T. Amano. 2010. Effects of landscape features on waterbird use of rice fields. Waterbirds 33:151-159. https://doi.org/10.1675/063.033.s111
Kittur, S., and K. S. G. Sundar. 2021. Of irrigation canals and multifunctional agroforestry: traditional agriculture facilitates Woolly-necked Stork breeding in a north Indian agricultural landscape. Global Ecology and Conservation 30:e01793. https://doi.org/10.1016/j.gecco.2021.e01793
Koju, R., B. Maharjan, K. R. Gosai, S. Kittur, and K. S. G. Sundar. 2019. Ciconiiformes nesting on trees in cereal-dominated farmlands: importance of scattered trees for heronries in lowland Nepal. Waterbirds 42:355-453. https://doi.org/10.1675/063.042.0401
Kushwaha, S., and A. Kumar. 2018. Occurrence and distribution of stork species in Bundelkhand Region, the drought prone landscape of India. International Journal of Trend in Scientific Research and Development 3:958-965. https://doi.org/10.31142/ijtsrd19177
Lowder, S. K., J. Skoet, and T. Raney. 2016. The number, size, and distribution of farms, smallholder farms, and family farms worldwide. World Development 87:16-29. https://doi.org/10.1016/j.worlddev.2015.10.041
Manley, B. F. J., L. L. McDonald, D. L. Thomas, T. L. McDonald, and W. P. Erickson. 2004. Resource selection by animals: statistical design and analysis for field studies. Second edition. Kluwer Academic Publishers, Dordrecht, Netherlands.
Minias, P., K. Gach, R. Wlodarczyk, M. Bartos, J. Drzewińska-Chańko, M. Rembowski, D. Jakubas, and T. Janiszewski. 2020. Colony size as a predictor of breeding behaviour in a common waterbird. PLoS ONE 15:e0241602. https://doi.org/10.1371/journal.pone.0241602
Ministry of Forests and Environment (MFE). 2019. National level forests and land cover analysis of Nepal using Google Earth Images. Forest Research and Training Centre, Kathmandu, Nepal. http://frtc.gov.np/old/downloadfile/Forest%20and%20Land%20Cover%20Analysis_final_report_1550056440.pdf
Mishra, A., J. N. Mandal, and T. K. Ghosh. 2004. Breeding of Lesser Adjutant from an unexplored area of Kosi region of N. Bihar. Newsletter for Birdwatchers 44(6):84.
Mitra, A., C. Chatterjee, and F. B. Mandal. 2011. Synthetic chemical pesticides and their effects on birds. Research Journal of Environmental Toxicology 5(2):81-96. https://doi.org/10.3923/rjet.2011.81.96
Onmuş, O., Y. Aǧaoǧlu, and O. Gül. 2012. Environmental factors affecting nest-site selection and breeding success of the white stork (Ciconia ciconia) in western Turkey. Wilson Journal of Ornithology 124:354-361. https://doi.org/10.1676/11-155.1
Pan-European Common Bird Monitoring Scheme(PECBMS). 2020. European wild bird indicators, 2020 update. Czech Society for Ornithology, Prague Czech Republic. https://pecbms.info/european-wild-bird-indicators-2020-update/
Paradis, E., and K. Schliep. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses. Bioinformatics 35:526-528. https://doi.org/10.1093/bioinformatics/bty633
Pearce-Higgins, J. W., S. M. Eglington, B. Martay, and D. E. Chamberlain. 2015. Drivers of climate change impacts on bird communities. Journal of Animal Ecology 84:943-954. https://doi.org/10.1111/1365-2656.12364
Poudyal, L. P., and S. Nepal. 2010. Population status of Lesser Adjutant in Chitwan National Park, Nepal. Danphe 19:1-3.
R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org.
Reif, J., and Z. Vermouzek. 2019. Collapse of farmland bird populations in an Eastern European country following its EU accession. Conservation Letters 12:1-8. https://doi.org/10.1111/conl.12585
Ricciardi, V., Z. Mehrabi, H. Wittman, D. James, and N. Ramankutty. 2021. Higher yields and more biodiversity on smaller farms. Nature Sustainability 4:651-657. https://doi.org/10.1038/s41893-021-00699-2
Rimal, B., R. Sharma, R. Kunwar, H. Keshtkar, N. E. Stork, S. Rijal, S. A. Rahman, and H. Baral. 2019. Effects of land use and land cover change on ecosystem services in the Koshi River Basin, Eastern Nepal. Ecosystem Services 38:100963. https://doi.org/10.1016/j.ecoser.2019.100963
Rimal, B., L. Zhang, H. Keshtkar, B. Haack, S. Rijal, and P. Zhang. 2018b. Land use/land cover dynamics and modeling of urban land expansion by the integration of Cellular Automata and Markov Chain. ISPRS International Journal of Geo-Information 7:154. https://doi.org/10.3390/ijgi7040154
Rimal, B., L. Zhang, N. Stork, S. Sloan, and S. Rijal. 2018a. Urban expansion occurred at the expense of agricultural lands in the Tarai region of Nepal from 1989 to 2016. Sustainability 10:1341. https://doi.org/10.3390/su10051341
Rokka, P., S. Bajagain, A. Pradhan, and M. Maharjan. 2021. The first nesting record of the Lesser Adjutant from Rautahat District of Nepal. Zoo’s Print 36:12-15. https://zoosprint.zooreach.org/index.php/zp/article/view/7210/6542
Schneider, A. 2012. Monitoring land cover change in urban and peri-urban areas using dense time stacks of Landsat satellite data and a data mining approach. Remote Sensing of Environment 124:689-704. https://doi.org/10.1016/j.rse.2012.06.006
Serrano, D., D. Oro, E. Ursúa, and J. L. Tella. 2005. Colony size selection determines adult survival and dispersal preferences: Allee effects in a colonial bird. American Naturalist 166:e22-e31. https://doi.org/10.1086/431255
Sharma, S. 2006. Population status and distribution of Lesser Adjutant (Leptoptilos javanicus) in far-western lowland Nepal. Tigerpaper 33:9-11. https://www.fao.org/3/ak863e/ak863e.pdf
Sheeba, N., and L. Vijayan. 2011. Sighting of the Lesser Adjutant Leptoptilos javanicus at Uppalapadu Heronry, Andhra Pradesh, India. Zoo’s Print 26:26. https://www.zoosprint.zooreach.org/index.php/zp/article/view/1674
Sreekar, R., A. Naidu, M. Seetharamaraju, and C. Srinivasulu. 2010. Lesser Adjutant Stork and Stork-billed Kingfisher, additions to the birds of Kawal Wildlife Sanctuary, Andhra Pradesh. Indian Birds 6:163-164. http://www.indianbirds.in/pdfs/Sreekar%20et%20al_Kawal.pdf
Stanton, R. L., C. A. Morrissey, and R. G. Clark. 2018. Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agriculture, Ecosystems & Environment 254:244-254. https://doi.org/10.1016/j.agee.2017.11.028
Subaraj, R., and A. F. S. L. Lok. 2009. Status of the Lesser Adjutant Stork (Leptoptilos Javanicus) in Singapore. Nature in Singapore 2:107-113.
Sundar, K. S. G. 2009. Are rice paddies suboptimal breeding habitat for Sarus Cranes in Uttar Pradesh, India? Condor 111:611-623. https://doi.org/10.1525/cond.2009.080032
Sundar, K. S. G. 2011. Agricultural intensification, rainfall patterns, and large waterbird breeding success in the extensively cultivated landscape of Uttar Pradesh, India. Biological Conservation 144:3055-3063. https://doi.org/10.1016/j.biocon.2011.09.012
Sundar, K. S. G., and S. A. Kittur. 2012. Methodological, temporal and spatial factors affecting modeled occupancy of resident birds in the perennially cultivated landscape of Uttar Pradesh, India. Landscape Ecology 27:59-71. https://doi.org/10.1007/s10980-011-9666-3
Sundar, K. S. G., R. Koju, B. Maharjan, B. G. Marcot, S. Kittur, and K. R. Gosai. 2019. First assessment of factors affecting the breeding success of two stork species in lowland Nepal using Bayesian network models. Wildfowl 69:45-69. https://www.fs.fed.us/pnw/pubs/journals/pnw_2019_sundar001.pdf
Sundar, K. S. G., B. Maharjan, R. Koju, S. Kittur, and K. R. Gosai. 2016. Factors affecting provisioning times of two stork species in lowland Nepal. Waterbirds 39:365-374. https://doi.org/10.1675/063.039.0406
Tourenq, C., S. Benhamou, N. Sadoul, A. Sandoz, F. Mesléard, J. L. Martin, and H. Hafner. 2004. Spatial relationships between tree-nesting heron colonies and rice fields in the Camargue, France. Auk 121:192-202.
Vergara, P., J. I. Aguirre, J. A. Fargallo, and J. A. Dávila. 2006. Nest-site fidelity and breeding success in White Stork Ciconia ciconia. Ibis 148:672-677. https://doi.org/10.1111/j.1474-919X.2006.00565.x
Wagle, Y., B. P. Bhattarai, and J. N. Adhikari. 2022. Factors influencing distribution and habitat utilisation of Leptoptilos javanicus in and around Barandabhar Corridor Forest, Chitwan, Nepal. Nature Conservation Research 7:19-26. https://doi.org/10.24189/ncr.2022.005
Wilke, C. O. 2021. ggridges: Ridgeline Plots in “ggplot2.” R package version 0.5.3. https://CRAN.R-project.org/package=ggridges
Fig. 1

Fig. 1. Lesser Adjutants (Leptoptilos javanicus) in the study area, as follows: (a) adult in rice field beside human habitation, (b) a colony in a Bombax ceiba tree within an agricultural landscape, and (c) fledglings at two nests.

Fig. 2

Fig. 2. Surveyed locations for Lesser Adjutant Stork (Leptoptilos javanicus) breeding colonies in lowland Nepal (country map) and details of locations where colonies were discovered. Detailed maps show land use, location of colonies, and locations of points that were used to measure available tree species on each landscape. We did not find colonies in Kailali and Chitwan districts (shaded areas).

Fig. 3

Fig. 3. Ridgeline density plots comparing metrics of trees used by Lesser Adjutant Storks (Leptoptilos javanicus) for nesting with metrics of available trees measured at random or systematic locations and jittered points of actual measurements. Graphs show comparisons for all trees measured (a, b) and only for Bombax ceiba (c, d). The different landscapes of lowland Nepal where the study was carried out are on the y-axis, as follows: UDAY—Udayapur, SARL—Sarlahi, MOJH—Morang-Jhapa, KARU— Kapilvastu-Rupandehi, and BARA—Bara.

Fig. 4

Fig. 4. The effect of site-level variables (tree height and location of nest tree; human habitation or non-human habitation), colony-level variables (colony size and chicks per nest) and landscape-level variables (wetland area, human habitation area, and distance to nearest wetland) measured around the colony on breeding ecology of the Lesser Adjutant (Leptoptilos javanicus) in lowland Nepal. The three graphs are, (a) colony site selection, (b) colony size, and (c) fledgling success. The standardized coefficients were calculated from the full model averaging method. Horizontal blue lines represent 95% confidence intervals. The variable was considered significant or relatively strong when its 95% CI did not with overlap with zero (dashed vertical lines).

Table 1
Table 1. Summary of studies conducted on Lesser Adjutants (Leptoptilos javanicus). We summarized the studies conducted on nesting and breeding ecology into the following three groups: (1) studies with basic natural history findings such as first record or identifying nest sites or habitat use or short distribution review within the country, (2) studies providing some metrics of breeding variables such as nest/colony size, number of chicks, and fledgling success, and (3) studies with hypothesis testing that explained factors affecting the nesting site or breeding success.
| Summarized topic | Location | Habitat use | Major findings | References |
| 1. Basic observations | ||||
| First locality records, distribution, and nesting tree used | Nepal, India, Bhutan, Sri Lanka, Singapore | Agricultural land, wetland, forest, and savanna | Confirmed first species record either from the specific locality or country level; showed distribution, habitat utilization, and tree species used by the stork. | Choudhury 2005, Sharma 2006, Subaraj and Lok 2009, Sreekar et al. 2010, Sheeba and Vijayan 2011, Bandara and Ranawana 2014, de Silva et al. 2015, Kushwaha and Kumar 2018, Wagle et al. 2022 |
| 2. Studies with some metrics of breeding variables | ||||
| Nesting and breeding confirmation | Nepal, India | Agricultural land, forest, mangrove, grassland/shrubland, riverine forest and grassland | Identified nesting sites, tree species, tree height, nest number, or total chicks; however, there was no information on the fledgling or breeding success as the study was conducted 1–3 times. Nesting tree reported in Nepal were Bombax ceiba, Ficus religiosa, and Dalbergia sissoo and, in India, were Ficus benghalensis, Ficus religiosa, Mangifera indica, and Bombax ceiba. | Mishra et al. 2004, Gopi and Pandav 2007, Poudyal and Nepal 2010, Dwivedi et al. 2013, Bajagain and Pradhan 2018, Bajagain et al. 2019, Baral et al. 2020, Rokka et al. 2021 |
| Discovery of breeding colony based on multiple years (2008–2012) | Bangladesh | Human settlement | Evaluated fledgling success for four years and identifies habitat use, tree species, and tree height. The tree species used was Bombax ceiba. | Chowdhury and Sourav 2012 |
| Nest site selection, population status, and conservation challenges | Central, Eastern Nepal | Agriculture land, riverine forest, grassland, forest, human settlement, and road | The number of nest was positively correlated with tree height, DBH, and canopy. Distance to human settlements, wetlands and roads influenced nesting colonies. Tree species used were Bombax ceiba, Haldina cordifolia, Ficus racemosa, Shorea robusta, and Terminalia alata. Wetland exploitation and human disturbance were the major threats. | Karki and Thapa 2013, Bhattarai et al. 2021 |
| 3. Detailed studies with some hypothesis testing | ||||
| Nesting tree and factors affecting breeding success and chicks provisioning | Central Nepal | Agricultural landscapes | Season and extent of wetland influenced the breeding success; model selection showed that different variables (seasons + wetland and human settlement area + colony size + chick age + brood size) affect food provisioning. Preferred nesting tree species were Bombax ceiba and Ficus religiosa | Sundar et al. 2016, 2019, Koju et al. 2019 |
Table 2
Table 2. Tree species used by breeding Lesser Adjutants (Leptoptilos javanicus) in different locations across lowland Nepal. Signs indicate trees that were used significantly more relative to availability (+), used in proportion to availability (0), and avoided or used significantly less relative to availability (‒). Trees that were not found in a particular locality do not have a sign. “Other” refers to the combination of all the tree species in a locality that did not have any stork colonies. Numbers of colonies monitored are indicated below location names. Significance was taken at P ≤ 0.05 level.
| Tree species | Bara (n = 8) |
Kapilvastu- Rupandehi (n = 29) |
Morang- Jhapa (n = 20) |
Sarlahi (n = 4) |
Udayapur (n = 4) |
| Albizia lucidior | + | ||||
| Bombax ceiba | ‒ | + | + | 0 | + |
| Breonia chinensis | ‒ | ||||
| Ficus religiosa | 0 | + | |||
| Haldinia cordifolia | + | ||||
| Mangifera indica | 0 | ||||
| Shorea robusta | 0 | + | |||
| Terminalia bellerica | + | ||||
| Trewia nudiflora | + | ||||
| Others | ‒ | ‒ | ‒ | ‒ | ‒ |